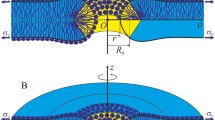
Abstract
The formation of through pores in lipid bilayer membranes occurs in a number of cellular processes and is also applied for biotechnological and biomedical purposes. In the classical theory of pore formation, diffusion of membrane defects in space of radii is considered. When the first pore reaches a critical radius, the membrane irreversibly ruptures. It is usually presumed that the diffusion of defects occurs independently; their possible lateral interactions are not taken into account. In this paper, we consider a possible influence of lateral interactions of metastable through pores on their kinetics. It is assumed that the interaction occurs due to the overlap of elastic deformation fields arising at the edges of two pores. The interaction energy of two circular pores was calculated in the Derjaguin approximation for rapidly decaying potentials. The unidimensional potential of interaction of two linear parallel edges of pores formed in membranes of different lipid composition was calculated in the framework of the theory of elasticity of liquid crystals adapted to lipid membranes. It is shown that this interaction should lead to a considerable reduction of the measured line tension of the pore edge. In addition, the lifetime of two optimally situated metastable pores can increase approximately 10 times due to the interaction. Extrapolation of the obtained results to the case of a larger number of interacting pores makes it possible to predict an additional increase in the lifetime by one or two orders of magnitude.



Similar content being viewed by others
REFERENCES
Davis H.T. 1996. Statistical mechanics of phases, interfaces, and thin films. New York: Wiley-VCH.
Cass A., Finkelstein A. 1967. Water permeability of thin lipid membranes. J. Gen. Physiol. 50, 1765–1784.
Peterlin P., Arrigler V., Diamant H., Haleva E. 2012. Permeability of phospholipid membrane for small polar molecules determined from osmotic swelling of giant phospholipid vesicles. Advances in Planar Lipid Bilayers and Liposomes.16, 301–335.
Nicholls D. 2013. Bioenergetics. 4th ed. Amsterdam: Academic Press.
Renault T.T., Floros K.V., Elkholi R., Corrigan K.A., Kushnareva Y., Wieder S.Y., Lindtner C., Serasinghe M.N., Asciolla J.J., Buettner C., Newmeyer D.D., Chipuk J.E. 2015. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol. Cell.57, 69–82.
Basañez G., Sharpe J.C., Galanis J., Brandt T.B., Hardwick J.M., Zimmerberg J. 2002. BAX-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J. Biol. Chem. 277, 49360–49365.
Li C., Salditt T. 2006. Structure of magainin and alamethicin in model membranes studied by X-ray reflectivity. Biophys. J.91, 3285–3300.
Sychev S.V., Balandin S.V., Panteleev P.V., Barsukov L.I., Ovchinnikova T.V. 2015. Lipid-dependent pore formation by antimicrobial peptides arenicin-2 and melittin demonstrated by their proton transfer activity. J. Pept. Sci. 21, 71–76.
Karal M.A.S., Alam J.M., Takahashi T., Levadny V., Yamazaki M. 2015. Stretch-activated pore of the antimicrobial peptide, magainin 2. Langmuir31, 3391–3401.
Cervia L.D., Chang C.C., Wang L., Mao M., Yuan F. 2018. Enhancing electrotransfection efficiency through improvement in nuclear entry of plasmid DNA. Mol. Ther. Nucleic Acids11, 263–271.
Pavlin M., Kandušer M. 2015. New insights into the mechanisms of gene electrotransfer–experimental and theoretical analysis. Sci. Rep. 5, 9132.
Karpunin D.V., Akimov S.A., Frolov V.A. 2005. Pore formation in lipid membranes containing lysolipids and cholesterol. Biol. Membrany (Rus.). 22, 429–432.
Bruen D., Delaney C., Florea L., Diamond D. 2017. Glucose sensing for diabetes monitoring: Recent developments. Sensors.17, 1866.
Evans E., Smith B.A. 2011. Kinetics of hole nucleation in biomembrane rupture. New J. Phys. 13, 095010.
Karal M.A.S., Yamazaki M. 2015. Communication: Activation energy of tension-induced pore formation in lipid membranes. J. Chem. Phys. 143, 081103.
Karal M.A.S., Levadnyy V., Yamazaki M. 2016. Analysis of constant tension-induced rupture of lipid membranes using activation energy. Phys. Chem. Chem. Phys. 18, 13487–13495.
Evans E., Heinrich V., Ludwig F., Rawicz W. 2003. Dynamic tension spectroscopy and strength of biomembranes. Biophys. J.85, 2342–2350.
Melikov K.C., Frolov V.A., Shcherbakov A., Samsonov A.V., Chizmadzhev Y.A., Chernomordik L.V. 2001. Voltage-induced nonconductive pre-pores and metastable single pores in unmodified planar lipid bilayer. Biophys. J.80, 1829–1836.
Abidor I.G., Arakelyan V.B., Chernomordik L.V., Chizmadzhev Y.A., Pastushenko V.F., Tarasevich M.R. 1979. Electric breakdown of bilayer lipid membranes I. The main experimental facts and their qualitative discussion. J. Electroanal. Chem. 104, 37–52.
Panov P.V., Akimov S.A., Batishchev O.V. 2014. Isoprenoid lipid chains increase membrane resistance to pore formation. Biol. Membrany (Rus.). 31, 331–335.
Derjaguin B.V. 1989. Theory of stability of colloids and thin films. Springer US. ISBN 978-0-306-11022-1.
Portet T., Dimova R. 2010. A new method for measuring edge tensions and stability of lipid bilayers: Effect of membrane composition. Biophys. J.99, 3264–3273.
Rawicz W., Olbrich K.C., McIntosh T., Needham D., Evans E. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J.79, 328–339.
Helfrich W. 1973. Elastic properties of lipid bilayers: Theory and possible experiments. Z. Naturforsch. C.28, 693–703.
Akimov S.A., Volynsky P.E., Galimzyanov T.R., Kuzmin P.I., Pavlov K.V., Batishchev O.V. 2017. Pore formation in lipid membrane I: Continuous reversible trajectory from intact bilayer through hydrophobic defect to transversal pore. Sci. Rep. 7, 12152.
Akimov S.A., Volynsky P.E., Galimzyanov T.R., Kuzmin P.I., Pavlov K.V., Batishchev O.V. 2017. Pore formation in lipid membrane II: Energy landscape under external stress. Sci. Rep. 7, 12509.
Markin V.S., Kozlov M.M. 1985. Pores statistics in bilayer lipid-membranes. Biol. Membrany (Rus.). 2, 205–223.
Pastushenko V.F., Chizmadzhev Y.A., Arakelyan V.B. 1979. Electric breakdown of bilayer lipid membranes II. Calculation of the membrane lifetime in the steady-state diffusion approximation. J. Electroanal. Chem. 104, 37–52.
Weaver J.C., Chizmadzhev Y.A. 1996. Theory of electroporation: A review. Bioelectrochem. Bioenerg. 41, 135–160.
Landau L.D., Lifshitz E.M., Pitaevskij L.P. 1981. Course of theoretical physics. Vol. 10: Physical kinetics. Oxford.
Awasthi N., Hub J.S. 2016. Simulations of pore formation in lipid membranes: Reaction coordinates, convergence, hysteresis, and finite-size effects. J. Chem. Theory Comput. 12, 3261–3269.
Kirsch S.A., Böckmann R.A. 2016. Membrane pore formation in atomistic and coarse-grained simulations. Biochim. Biophys. Acta. 1858, 2266–2277.
Molotkovsky R.J., Akimov S.A. 2009. Calculation of the line tension in various models of the lipid bilayer pore edge. Biol. Membrany (Rus.). 26, 149–158.
Galimzyanov T.R., Molotkovsky R.J., Kuzmin P.I., Akimov S.A. 2011. Stabilization of the raft bilayer structure due to elastic deformations of the membrane. Biol. Membrany (Rus.). 28, 307–314.
Akimov S.A., Frolov V.A., Kuzmin P.I., Zimmerberg J., Chizmadzhev Y.A., Cohen F.S. 2008. Domain formation in membranes caused by lipid wetting of protein. Phys. Rev. E.77, 051901.
Galimzyanov T.R., Molotkovsky R.J., Kheyfets B.B., Akimov S.A. 2013. Energy of the interaction between membrane lipid domains calculated from splay and tilt deformations. JETP Lett. 96, 681–686.
Akimov S.A., Aleksandrova V.V., Galimzyanov T.R., Batishchev O.V. 2017. Interaction of amphipathic peptides mediated by elastic membrane deformations. Biol. Membrany (Rus.). 34, 162–173.
Kondrashov O.V., Galimzyanov T.R., Pavlov K.V., Kotova E.A., Antonenko Y.N., Akimov S.A. 2018. Membrane elastic deformations modulate gramicidin A transbilayer dimerization and lateral clustering. Biophys. J.115, 478–493.
Kondrashov O.V., Galimzyanov T.R., Jiménez-Munguía I., Batishchev O.V., Akimov S.A. 2019. Membrane-mediated interaction of amphipathic peptides can be described by a one-dimensional approach. Phys. Rev. E.99, 022401.
Israelachvili J. 2011. Intermolecular and surface forces. New York: Academic Press.
Hamm M., Kozlov M.M. 2000. Elastic energy of tilt and bending of fluid membranes. Eur. Phys. J. E.3, 323–335.
Leikin S., Kozlov M.M., Fuller N.L., Rand R.P. 1996. Measured effects of diacylglycerol on structural and elastic properties of phospholipid membranes. Biophys. J.71, 2623–2632.
Nagle J.F., Wilkinson D.A. 1978. Lecithin bilayers. Density measurement and molecular interactions. Biophys. J.23, 159–175.
Dimova R. 2014. Recent developments in the field of bending rigidity measurements on membranes. Adv. Colloid Interface Sci.208, 225–234.
Hamm M., Kozlov M.M. 1998. Tilt model of inverted amphiphilic mesophase. Eur. Phys. J. B.6, 519–528.
Bennett W.D., Sapay N., Tieleman D.P. 2014. Atomistic simulations of pore formation and closure in lipid bilayers. Biophys. J.106, 210–219.
Wohlert J., den Otter W.K., Edholm O., Briels W.J. 2006. Free energy of a trans-membrane pore calculated from atomistic molecular dynamics simulations. J. Chem. Phys. 124, 154905.
ACKNOWLEDGMENTS
The work was supported by the Ministry of Science and Higher Education of the Russian Federation and partially by the Russian Foundation for Basic Research (project nos. 17-04-02070 and 18-54-74001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by R. Molotkovsky
Rights and permissions
About this article
Cite this article
Galimzyanov, T.R., Molotkovsky, R.J., Kalutsky, M.A. et al. Lateral Interactions Influence the Kinetics of Metastable Pores in Lipid Membranes. Biochem. Moscow Suppl. Ser. A 14, 117–125 (2020). https://doi.org/10.1134/S1990747820010055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747820010055