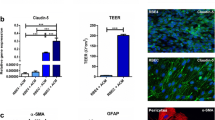
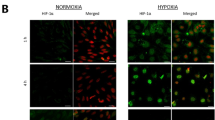
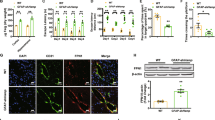
Abstract
Ferroportin plays an essential role for iron transport through the blood-brain barrier (BBB), which is formed by brain capillary endothelial cells (BCECs). To maintain the integrity of the BBB, the BCECs gain support from pericytes and astrocytes, which together with neurons form the neurovascular unit (NVU). The objectives of the present study were to investigate ferroportin expression in primary cells of the NVU and to determine if ferroportin mRNA (Fpn) expression is epigenetically regulated. Primary rat BCECs, pericytes, astrocytes, and neurons all expressed ferroportin mRNA at varying levels, with BCECs exhibiting the highest expression of Fpn, peaking when co-cultured but examined separately from astrocytes. Conversely, Fpn expression was lowest in isolated astrocytes, which correlated with high DNA methylation in their Slc40a1 promoter. To provide further evidence for epigenetic regulation, mono-cultured BCECs, pericytes, and astrocytes were treated with the histone deacetylase inhibitors valproic acid (VPA) and sodium butyrate (SB), which significantly increased Fpn and ferroportin protein in BCECs and pericytes. Furthermore, 59Fe export from BCECs was elevated after treatment with VPA. In conclusion, we present first time evidence stating that Fpn expression is epigenetically regulated in BCECs, which may have implications for pharmacological induction of iron transport through the BBB.





Similar content being viewed by others
Abbreviations
- 5mC:
-
5-Methylcytosine
- α-SMA:
-
α-Smooth actin
- Actb:
-
β-Actin
- ARE:
-
Antioxidant responsive element
- BACH1:
-
BTB and CNC homology 1
- BBB:
-
Blood-brain barrier
- BCECs:
-
Brain capillary endothelial cells
- BSA:
-
Bovine serum albumin
- cDNA:
-
Complementary DNA
- CG:
-
Cytosine-guanine
- DAPI:
-
4′,6-diamidino-2-phenylindole
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- DMT1:
-
Divalent metal transporter 1
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- FCS:
-
Fetal calf serum
- Fe:
-
Iron
- Fpn:
-
Ferroportin
- GFAP:
-
Glial fibrillary acidic protein
- HDAC:
-
Histone deacetylase
- HDACi:
-
Histone deacetylase inhibitor
- IRE:
-
Iron responsive element
- IRP:
-
Iron regulatory protein
- NRF2:
-
Nuclear factor (erythroid-derived 2)-like-2
- NVU:
-
Neurovascular unit
- PBS:
-
Phosphate-buffered saline
- SAM:
-
SAdenosylmethionine
- SB:
-
Sodium butyrate
- TfR1:
-
Transferrin receptor 1
- VPA:
-
Valproic acid
- TEER:
-
Trans-endothelial electrical resistance
- TSS:
-
Transcription start site
- TU-20:
-
β3 tubulin
- ZO-1:
-
Zonula occludens protein 1
References
Abbott NJ (2013) Blood–brain barrier structure and function and the challenges for CNS drug delivery. J Inherit Metab Dis 36:437–449. https://doi.org/10.1007/s10545-013-9608-0
Mills E, Dong X-P, Wang F, Xu H (2010) Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med Chem 2:51–64. https://doi.org/10.1021/ac901991x
Duck KA, Connor JR (2016) Iron uptake and transport across physiological barriers. BioMetals 29:573–591. https://doi.org/10.1007/s10534-016-9952-2
Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25. https://doi.org/10.1016/j.nbd.2009.07.030
McConnell HL, Kersch CN, Woltjer RL et al (2016) The translational significance of the neurovascular unit. JBC Pap. https://doi.org/10.1074/jbc.R116.760215
Thomsen MS, Routhe LJ, Moos T (2017) The vascular basement membrane in the healthy and pathological brain. J Cereb Blood Flow Metab 37:3300–3317. https://doi.org/10.1177/0271678X17722436
Burkhart A, Skjørringe T, Johnsen KB, Siupka P, Thomsen LB, Nielsen MS, Thomsen LL, Moos T (2016) Expression of iron-related proteins at the neurovascular unit supports reduction and reoxidation of iron for transport through the blood-brain barrier. Mol Neurobiol 53:7237–7253. https://doi.org/10.1007/s12035-015-9582-7
Moos T, Rosengren Nielsen T, Skjørringe T, Morgan EH (2007) Iron trafficking inside the brain. J Neurochem 103:1730–1740. https://doi.org/10.1111/j.1471-4159.2007.04976.x
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139:179–197. https://doi.org/10.1111/jnc.13425
Abboud S, Haile DJ (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J Biol Chem 275:19906–19912. https://doi.org/10.1074/jbc.M000713200
Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt SJ, Moynihan J, Paw BH, Drejer A et al (2000) Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature 403:776–781. https://doi.org/10.1038/35001596
McKie AT, Marciani P, Rolfs A et al (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell 5:299–309
Wallace DF (2016) The regulation of iron absorption and homeostasis. Clin Biochem Rev 37:51–62
Nemeth E, Tuttle MS, Powelson J et al (2004) Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science (80-) 306:2090–2093. https://doi.org/10.1126/science.1104742
Donovan A, Lima CA, Pinkus JL, Pinkus GS, Zon LI, Robine S, Andrews NC (2005) The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab 1:191–200. https://doi.org/10.1016/j.cmet.2005.01.003
Rochette L, Gudjoncik A, Guenancia C, Zeller M, Cottin Y, Vergely C (2015) The iron-regulatory hormone hepcidin: a possible therapeutic target? Pharmacol Ther 146:35–52. https://doi.org/10.1016/j.pharmthera.2014.09.004
Le Gac G, Ka C, Joubrel R et al (2013) Structure-function analysis of the human ferroportin iron exporter (SLC40A1): effect of hemochromatosis type 4 disease mutations and identification of critical residues. Hum Mutat 34:1371–1380. https://doi.org/10.1002/humu.22369
Ward DM, Kaplan J (2012) Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta, Mol Cell Res 1823:1426–1433. https://doi.org/10.1016/j.bbamcr.2012.03.004
Bonaccorsi di Patti MC, Polticelli F, Cece G, Cutone A, Felici F, Persichini T, Musci G (2014) A structural model of human ferroportin and of its iron binding site. FEBS J 281:2851–2860. https://doi.org/10.1111/febs.12825
Andersen HH, Johnsen KB, Moos T (2014) Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell Mol Life Sci 71:1607–1622. https://doi.org/10.1007/s00018-013-1509-8
Clardy SL, Wang X, Boyer PJ, Earley CJ, Allen RP, Connor JR (2006) Is ferroportin–hepcidin signaling altered in restless legs syndrome? J Neurol Sci 247:173–179. https://doi.org/10.1016/J.JNS.2006.04.008
Boserup MW, Lichota J, Haile D, Moos T (2011) Heterogenous distribution of ferroportin-containing neurons in mouse brain. BioMetals 24:357–375. https://doi.org/10.1007/s10534-010-9405-2
Wu LJC, Leenders AGM, Cooperman S, Meyron-Holtz E, Smith S, Land W, Tsai RYL, Berger UV et al (2004) Expression of the iron transporter ferroportin in synaptic vesicles and the blood-brain barrier. Brain Res 1001:108–117. https://doi.org/10.1016/j.brainres.2003.10.066
Muckenthaler MU, Rivella S, Hentze MW, Galy B (2017) A red carpet for iron metabolism. Cell 3:1–18. https://doi.org/10.1016/j.cell.2016.12.034
Drakesmith H, Nemeth E, Ganz T (2015) Ironing out ferroportin. Cell Metab 22:777–787. https://doi.org/10.1016/j.cmet.2015.09.006
Marro S, Chiabrando D, Messana E, Stolte J, Turco E, Tolosano E, Muckenthaler MU (2010) Heme controls ferroportin1 (FPN1) transcription involving Bach1, Nrf2 and a MARE/ARE sequence motif at position -7007 of the FPN1 promoter. Haematologica 95:1261–1268. https://doi.org/10.3324/haematol.2009.020123
Silva B, Faustino P (2015) An overview of molecular basis of iron metabolism regulation and the associated pathologies. Biochim Biophys Acta 1852:1347–1359. https://doi.org/10.1016/j.bbadis.2015.03.011
Zhang D-L, Hughes RM, Ollivierre-Wilson H, Ghosh MC, Rouault TA (2009) A ferroportin transcript that lacks an iron-responsive element enables duodenal and erythroid precursor cells to evade translational repression. Cell Metab 9:461–473. https://doi.org/10.1016/j.cmet.2009.03.006
Gulec S, Anderson GJ, Collins JF (2014) Mechanistic and regulatory aspects of intestinal iron absorption. AJP Gastrointest Liver Physiol 307:G397–G409. https://doi.org/10.1152/ajpgi.00348.2013
Helgudottir SS, Lichota J, Burkhart A, Moos T (2018) Hepcidin mediates transcriptional changes in ferroportin mRNA in differentiated neuronal-like PC12 cells subjected to iron challenge. Mol Neurobiol 56:2362–2374 3–5
Rishi G, Wallace DF, Subramaniam VN (2015) Hepcidin: regulation of the master iron regulator. Biosci Rep 35:e00192. https://doi.org/10.1042/BSR20150014
Wang L, Liu X, You L-H, Ci YZ, Chang S, Yu P, Gao G, Chang YZ (2019) Hepcidin and iron regulatory proteins coordinately regulate ferroportin 1 expression in the brain of mice. J Cell Physiol 234:7600–7607. https://doi.org/10.1002/jcp.27522
Chen Y, Zhang S, Wang X, Guo W, Wang L, Zhang D, Yuan L, Zhang Z et al (2015) Disordered signaling governing ferroportin transcription favors breast cancer growth. Cell Signal 27:168–176. https://doi.org/10.1016/j.cellsig.2014.11.002
Guo JU, Ma DK, Mo H, Ball MP, Jang MH, Bonaguidi MA, Balazer JA, Eaves HL et al (2011) Neuronal activity modifies the DNA methylation landscape in the adult brain. Nat Neurosci 14:1345–1351. https://doi.org/10.1038/nn.2900
Kohli RM, Zhang Y (2013) TET enzymes, TDG and the dynamics of DNA demethylation. Nature 502:472–479. https://doi.org/10.1038/nature12750
Yao B, Christian KM, He C, Jin P, Ming GL, Song H (2016) Epigenetic mechanisms in neurogenesis. Nat Rev Neurosci 17:537–549. https://doi.org/10.1038/nrn.2016.70
Tough DF, Tak PP, Tarakhovsky A, Prinjha RK (2016) Epigenetic drug discovery: breaking through the immune barrier. Nat Rev Drug Discov 15:835–853. https://doi.org/10.1038/nrd.2016.185
Benayoun BA, Pollina EA, Brunet A (2015) Epigenetic regulation of ageing: linking environmental inputs to genomic stability. Nat Rev Mol Cell Biol 16:593–610. https://doi.org/10.1038/nrm4048
Milutinovic S, Detich N, Szyf M (2007) Valproate induces widespread epigenetic reprogramming which involves demethylation of specific genes. Carcinogenesis 28:560–571. https://doi.org/10.1093/carcin/bgl167
Li E, Zhang Y (2014) DNA methylation in mammals. Cold Spring Harb Perspect Biol 6:a019133. https://doi.org/10.1101/cshperspect.a019133
Rossaert E, Pollari E, Jaspers T, van Helleputte L, Jarpe M, van Damme P, de Bock K, Moisse M et al (2019) Restoration of histone acetylation ameliorates disease and metabolic abnormalities in a FUS mouse model. Acta Neuropathol Commun 7:107. https://doi.org/10.1186/s40478-019-0750-2
Konsoula Z, Barile FA (2012) Epigenetic histone acetylation and deacetylation mechanisms in experimental models of neurodegenerative disorders. J Pharmacol Toxicol Methods 66:215–220. https://doi.org/10.1016/j.vascn.2012.08.001
Saha RN, Pahan K (2006) HATs and HDACs in neurodegeneration: a tale of disconcerted acetylation homeostasis. Cell Death Differ 13:539–550. https://doi.org/10.1038/sj.cdd.4401769
Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS (2001) Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem 276:36734–36741. https://doi.org/10.1074/jbc.M101287200
Zhu M-M, Li H-L, Shi L-H, Chen XP, Luo J, Zhang ZL (2017) The pharmacogenomics of valproic acid. J Hum Genet 62:1009–1014. https://doi.org/10.1038/jhg.2017.91
Bennett SA, Tanaz R, Cobos SN, Torrente MP (2019) Epigenetics in amyotrophic lateral sclerosis: a role for histone post-translational modifications in neurodegenerative disease. Transl Res 204:19–30. https://doi.org/10.1016/J.TRSL.2018.10.002
Burkhart A, Skjørringe T, Andresen TL et al (2017) Transfection of primary brain capillary endothelial cells for protein synthesis and secretion of recombinant erythropoietin: a strategy to enable protein delivery to the brain. Cell Mol Life Sci 74:2467–2485. https://doi.org/10.1007/s00018-017-2501-5
Burkhart A, Azizi M, Thomsen MSS et al (2014) Accessing targeted nanoparticles to the brain: the vascular route. Curr Med Chem 21:4092–4099
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Burdo JR, Menzies SL, Simpson IA, Garrick LM, Garrick MD, Dolan KG, Haile DJ, Beard JL et al (2001) Distribution of divalent metal transporter 1 and metal transport protein 1 in the normal and Belgrade rat. J Neurosci Res 66:1198–1207. https://doi.org/10.1002/jnr.1256
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163:1064–1078. https://doi.org/10.1016/j.cell.2015.10.067
Winkler EA, Sagare AP, Zlokovic BV (2014) The pericyte: a forgotten cell type with important implications for Alzheimer’s disease? Brain Pathol 24:371–386. https://doi.org/10.1111/bpa.12152
Yang WM, Jung KJ, Lee MO, Lee YS, Lee YH, Nakagawa S, Niwa M, Cho SS et al (2011) Transient expression of iron transport proteins in the capillary of the developing rat brain. Cell Mol Neurobiol 31:93–99. https://doi.org/10.1007/s10571-010-9558-0
Dringen R, Bishop GM, Koeppe M, Dang TN, Robinson SR (2007) The pivotal role of astrocytes in the metabolism of iron in the brain. Neurochem Res 32:1884–1890. https://doi.org/10.1007/s11064-007-9375-0
Schipper HM, Vininsky R, Brull R, Small L, Brawer JR (1998) Astrocyte mitochondria: a substrate for iron deposition in the aging rat substantia nigra. Exp Neurol 152:188–196. https://doi.org/10.1006/exnr.1998.6854
McCarthy RC, Kosman DJ (2014) Glial cell ceruloplasmin and hepcidin differentially regulate iron efflux from brain microvascular endothelial cells. PLoS One 9:e89003. https://doi.org/10.1371/journal.pone.0089003
Urrutia P, Aguirre P, Esparza A, Tapia V, Mena NP, Arredondo M, González-Billault C, Núñez MT (2013) Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J Neurochem 126:541–549. https://doi.org/10.1111/jnc.12244
Browne EP, Dinc SE, Punska EC, Agus S, Vitrinel A, Erdag GC, Anderton DL, Arcaro KF et al (2014) Promoter methylation in epithelial-enriched and epithelial-depleted cell populations isolated from breast milk. J Hum Lact 30:450–457. https://doi.org/10.1177/0890334414548224
Yong-Quan Ng G, Yun-An L, Sobey C et al (2018) Epigenetic regulation of inflammation in stroke. Ther Adv Neurol Disord 11:1–30
Brissot P, Pietrangelo A, Adams PC, de Graaff B, McLaren CE, Loréal O (2018) Haemochromatosis. Nat Rev Dis Prim 4:399–408. https://doi.org/10.1038/nrdp.2018.16
Qing H, He G, Ly PTT, Fox CJ, Staufenbiel M, Cai F, Zhang Z, Wei S et al (2008) Valproic acid inhibits Aβ production, neuritic plaque formation, and behavioral deficits in Alzheimer’s disease mouse models. J Exp Med 205:2781–2789. https://doi.org/10.1084/JEM.20081588
You D, Shin HM, Mosaad F, Richardson JR, Aleksunes LM (2019) Brain region-specific regulation of histone acetylation and efflux transporters in mice. J Biochem Mol Toxicol e22318. https://doi.org/10.1002/jbt.22318
You D, Wen X, Gorczyca L, Morris A, Richardson JR, Aleksunes LM (2019) Increased MDR1 transporter expression in human brain endothelial cells through enhanced histone acetylation and activation of aryl hydrocarbon receptor signaling. Mol Neurobiol. https://doi.org/10.1007/s12035-019-1565-7
Belaidi AA, Gunn AP, Wong BX, Ayton S, Appukuttan AT, Roberts BR, Duce JA, Bush AI (2018) Marked age-related changes in brain Iron homeostasis in amyloid protein precursor knockout mice. Neurotherapeutics 15:1055–1062. https://doi.org/10.1007/s13311-018-0656-x
Lei P, Ayton S, Finkelstein DI, Spoerri L, Ciccotosto GD, Wright DK, Wong BXW, Adlard PA et al (2012) Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 18:291–295. https://doi.org/10.1038/nm.2613
Biermann J, Boyle J, Pielen A, Lagrèze WA (2011) Histone deacetylase inhibitors sodium butyrate and valproic acid delay spontaneous cell death in purified rat retinal ganglion cells. Mol Vis 17:395–403
Candido EPM, Reeves R, Davie JR (1978) Sodium butyrate inhibits histone deacetylation in cultured cells. Cell 14:105–113. https://doi.org/10.1016/0092-8674(78)90305-7
Minucci S, Zhu P, Kra OH et al (2001) Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20:6969–6978. https://doi.org/10.1093/emboj/20.24.6969
Chateauvieux S, Morceau F, Dicato M, Diederich M (2010, 2010) Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol:1–18. https://doi.org/10.1155/2010/479364
Nikolian VC, Dekker SE, Bambakidis T, Higgins GA, Dennahy IS, Georgoff PE, Williams AM, Andjelkovic AV et al (2018) Improvement of blood-brain barrier integrity in traumatic brain injury and hemorrhagic shock following treatment with valproic acid and fresh frozen plasma. Crit Care Med 46:e59–e66. https://doi.org/10.1097/CCM.0000000000002800
Ying G, Jing C, Li J, Wu C, Yan F, Chen JY, Wang L, Dixon BJ et al (2016) Neuroprotective effects of valproic acid on blood-brain barrier disruption and apoptosis-related early brain injury in rats subjected to subarachnoid hemorrhage are modulated by heat shock protein 70/matrix metalloproteinases and heat shock protein 70/AKT pathways. Neurosurgery 79:286–295. https://doi.org/10.1227/NEU.0000000000001264
Wang Z, Leng Y, Tsai L-K, Leeds P, Chuang DM (2011) Valproic acid attenuates blood–brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab 31:52–57. https://doi.org/10.1038/jcbfm.2010.195
Chang P, Williams AM, Bhatti UF, Biesterveld BE, Liu B, Nikolian VC, Dennahy IS, Lee J et al (2019) Valproic acid and neural apoptosis, inflammation, and degeneration 30 days after traumatic brain injury, hemorrhagic shock, and polytrauma in a swine model. J Am Coll Surg 228:265–275. https://doi.org/10.1016/j.jamcollsurg.2018.12.026
Nikolian VC, Dennahy IS, Higgins GA, et al (2018) Transcriptomic changes following valproic acid treatment promote neurogenesis and minimize secondary brain injury. In: Journal of Trauma and Acute Care Surgery. Lippincott Williams and Wilkins, pp 459–465
Zhang DL, Ghosh MC, Rouault TA (2014) The physiological functions of iron regulatory proteins in iron homeostasis-an update. Front Pharmacol 5(JUN):124
Acknowledgments
We thank Merete Fredsgaard and Hanne Krone Nielsen, Aalborg University, Denmark, for their excellent technical assistance. We also thank Lykke Christina Grubach, Clinical Diagnostics Department at Aalborg University Hospital, Denmark for providing technical instruments.
Funding
The present work has been supported by the Danish Multiple Sclerosis Society, “Kong Christian den Tiendes fond,” “Åse og Ejner Danielsens Fond,” and the Lundbeck Foundation Research Initiative on Brain Barriers and Drug Delivery (Grant no. R155-2013-14113).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Helgudottir, S.S., Routhe, L.J., Burkhart, A. et al. Epigenetic Regulation of Ferroportin in Primary Cultures of the Rat Blood-Brain Barrier. Mol Neurobiol 57, 3526–3539 (2020). https://doi.org/10.1007/s12035-020-01953-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01953-y