Abstract
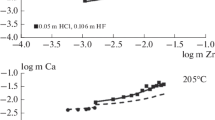
Metal complexes are thermally unstable in hydrothermal solutions, which may cause metal cation inertness or precipitation. We investigated, for the first time, the hydrolysis behavior of ammonium fluorozirconate ((NH4)2ZrF6) at 423.15–773.15 K and 100 MPa. Kinetic equilibration experiments of the fluorozirconate solution demonstrated that the hydrolysis of the complex reached equilibrium within 4 h. Variable-temperature experiments showed that the complex’s hydrolysis was dependent on the temperature and initial concentration in the hydrothermal solutions, with enhanced hydrolysis at elevated temperature and decreased initial concentrations. Based on our experimental data, we established a linear relationship between the cumulative hydrolysis constants (K) of (NH4)2ZrF6 and absolute temperature T: \(\ln K = \left( {36.06 \pm 3.46} \right) - \left( {29747 \pm 1967} \right)/T,\) from which \(\Delta_{{\text{r}}} H_{{\text{m}}}^{\Theta }\), \(\Delta_{{\text{r}}} S_{{\text{m}}}^{\Theta }\) and \(\Delta_{{\text{r}}} G_{{\text{m}}}^{\Theta }\) values for the hydrolysis reaction were also calculated. This study suggests that fluorine may play an important role in Zr mobility and provide a route to quantitatively characterize the thermodynamic features of metal complexes in hydrothermal solutions.




Similar content being viewed by others
References
Baes, J.C.F., Mesmer, R.E.: The thermodynamics of cation hydrolysis. Am. J. Sci. 281, 935–962 (1981)
Holovko, M., Druchok, M., Bryk, T.: Primitive model for cation hydrolysis: a molecular-dynamics study. J Chem. Phys. 123, 154505 (2005)
Holovko, M., Druchok, M., Bryk, T.: Cation Hydrolysis Phenomenon in Aqueous Solution: Towards Understanding It by Computer Simulations. Springer, Berlin (2009)
Considine, G.D.: Zirconium. Van Nostrand's Encyclopedia of Chemistry. Wiley, New York (2005)
Gieré, R.: Zirconolite, allanite and hoegbomite in a marble skarn from the Bergell contact aureole: implications for mobility of Ti. Zr and REE. Contrib. Mineral. Petrol. 93, 459–470 (1986)
Rubin, J.N., Henry, C.D., Price, J.G.: The mobility of zirconium and other “immobile” elements during hydrothermal alteration. Chem. Geol. 110, 29–47 (1993)
Ryzhenko, B.N., Kovalenko, N.I., Prisyagina, N.I., Starshinova, N.P., Krupskaya, V.V.: Experimental determination of zirconium speciation in hydrothermal solutions. Geochem. Int. 46, 328–339 (2008)
Migdisov, A.A., Williams-Jones, A.E., van Hinsberg, V., Salvi, S.: An experimental study of the solubility of baddeleyite (ZrO2) in fluoride-bearing solutions at elevated temperature. Geochim. Cosmochim. Acta 75, 7426–7434 (2011)
Hampson, G.C., Pauling, L.: The structure of ammonium heptafluozirconate and potassium heptafluozirconate and the configuration of the heptafluozirconate group. J. Am. Chem. Soc. 60, 2702–2707 (1938)
Krylov, A.S., Krylova, S.N., Laptash, N.M., Vtyurin, A.N.: Raman scattering study of temperature induced phase transitions in crystalline ammonium heptafluorozirconate, (NH4)3ZrF7. Vib. Spectrosc. 62, 258–263 (2012)
Nerád, I., Mikšíková, E., Kubíková, B.: Calorimetric investigation of tripotassium zirconate heptafluoride K3ZrF7. J. Mol. Liq. 290, 111191 (2019)
Griffith, W.P., Wickins, T.D.: Raman studies on species in aqueous solutions.Part II. Oxy-species of metals of Groups VIA, VA, and IVA. J. Chem. Soc. A 1967, 675–679 (1967)
Zalkin, A., Eimerl, D., Velsko, S.P.: Diammonium hexafluorozirconate. Acta Crystallogr. Sect. C 44, 2050–2051 (1988)
Bukvetskii, B.V., Gerasimenko, A.V., Davidovich, R.L.: Crystalline-structure of NH4ZrF5·0.75H2O and (NH4)2ZrF6 ammonium fluorozirconates. Koord. Khim. 17, 35–43 (1991)
He, J.J., Ding, X., Wang, Y.R., Sun, W.D.: The effects of precipitation-aging-re-dissolution and pressure on hydrolysis of fluorine-rich titanium complexes in hydrothermal fluids and its geological implications. Acta Petrol. Sin. 31, 1870–1878 (2015). (in Chinese with English abstract)
He, J.J., Ding, X., Wang, Y.R., Sun, W.D.: The effect of temperature and concentration on hydrolysis of fluorine-rich titanium complexes in hydrothermal fluids: Constraints on titanium mobility in deep geological processes. Acta Petrol. Sin. 31, 802–810 (2015). (in Chinese with English abstract)
Ding, X., Harlov, D.E., Chen, B., Sun, W.D.: Fluids, metals, and mineral/ore deposits. Geofluids 2018, 1–6 (2018)
Mo, J.X.: Impact on chemistry reaction speed, balance and conversion ratio of temperature. Higher Educ. Forum 5, 161–164 (2004). (In Chinese with English abstract)
Wang, Y., Chou, I.M.: Characteristics of hydrolysis of the complex Na2SnF6 in hydrothermal solutions: an experimental study. Chin. J. Geochem. 6, 372–382 (1987)
Wang, Y., Gu, F., Yuan, Z.: Partitioning and hydrolysis of Nb and Ta and their implications with regard to mineralization. Chin. J. Geochem. 12, 84–91 (1993)
Helgeson, H.C., Kirkham, D.H., Flowers, G.C.: Am. J. Sci. 281, 1249–1516 (1981)
Tanger, J.C., Helgeson, H.C.: Calculation of the thermodynamic and transport-properties of aqueous species at high-pressures and temperatures-revised equations of state for the standard partial molal properties of ions and electrolytes. Am. J. Sci. 288, 18–98 (1988)
Helgeson, H.C., Kirkham, D.H.: Theoretical prediction of the thermodynamic behavior of aqueous electrolytes at high pressures and temperatures; II, Debye-Hückel parameters for activity coefficients and relative partial molal properties. Am. J. Sci. 274, 1089–1198 (1974)
Garrels, R.M., Christ, C.L.: Solutions, Minerals, and Equilibria. Harper and Row, New York (1965)
Kielland, J.: Individual activity coefficients of ions in aqueous solutions. J. Am. Chem. Soc. 59, 1675–1678 (1937)
Aja, S.U., Wood, S.A., Williams-Jones, A.E.: The aqueous geochemistry of Zr and the solubility of some Zr-bearing minerals. Appl. Geochem. 10, 603–620 (1995)
Mysen, B.: An in situ experimental study of Zr4+ transport capacity of water-rich fluids in the temperature and pressure range of the deep crust and upper mantle. Prog. Earth Planet. Sci. 2, 38 (2015)
Schmidt, C., Rickers, K., Wirth, R., Nasdala, L.: Low-temperature Zr mobility: An in-situ synchrotron-radiation XRF study of the effect of radiation damage in zircon on the element release in H2O + HCl + SiO2 fluids. Am. Mineral. 91, 1211–1215 (2006)
Newton, R.C., Manning, C.E., Hanchar, J.M., Colasanti, C.V.: Free energy of formation of zircon based on solubility measurements at high temperature and pressure. Am. Mineral. 95, 52–58 (2010)
Ayers, J.C., Zhang, L., Luo, Y., Peters, T.J.: Zircon solubility in alkaline aqueous fluids at upper crustal conditions. Geochim. Cosmochim. Acta 96, 18–28 (2012)
Bernini, D., Audétat, A., Dolejš, D., Keppler, H.: Zircon solubility in aqueous fluids at high temperatures and pressures. Geochim. Cosmochim. Acta 119, 178–187 (2013)
Shikina, N.D., Vasina, O.N., Gurova, E.V., Popova, E.S., Tagirov, B.R., Shazzo, Y.K., Khodakovskii, I.L.: Experimental study of ZrO2(c) solubility in water and aqueous perchloric acid solutions at 150 and 250 °C. Geochem. Int. 52, 82–87 (2014)
Acknowledgements
This study was supported by the National Key R&D Program of China (2016YFC0600204; 2016YFC0600408), the National Natural Science Foundation of China (41773054) and the CAS Science Innovation Project for College Students. This is contribution No. IS-2874 from GIGCAS. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yan, H., He, J., Liu, X. et al. Thermodynamic Investigation of the Hydrolysis Behavior of Fluorozirconate Complexes at 423.15–773.15 K and 100 MPa. J Solution Chem 49, 836–848 (2020). https://doi.org/10.1007/s10953-020-00993-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-020-00993-1