Abstract
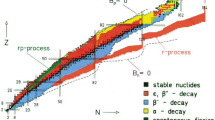
The year 2019 was proclaimed by the UN and UNESCO as the International Year of the Periodic Table of the Chemical Elements; by its beginning, the seventh period of the Table was already filled with new, very heavy elements. According to theoretical predictions, isotopes of superheavy elements with increased stability form a large zone in the form of an island with a vertex located near the “magic” numbers of protons Z = 114 and neutrons N = 184 on the nuclide map. New elements with atomic numbers from 114 to 118 were synthesized in the Flerov Laboratory of Nuclear Reactions, Joint Institute for Nuclear Research (JINR, Dubna), in 2000–2012 by fusion reactions of target nuclei (heavy actinide isotopes (Z = 94–98)) with bombarding 48Ca ions. The experimental results suggest that there may exist nuclei or elements with atomic numbers greater than 118 and mass greater than 300 amu. A new experimental complex, the Factory of Superheavy Elements (SHEF), was created in Dubna to study the nuclear and electronic structures of new elements and their chemical properties and to synthesize elements from the beginning of the eighth period of the Periodic Table. A new DC-280 accelerator has already been launched in the SHEF, and experiments are planned to begin. The introductory and final parts of my speech at the scientific session of the General Meeting of the Members of the Russian Academy of Sciences are related to the discovery of the Periodic Law published by D.I. Mendeleev 150 years ago. The action of this law in the properties of the heaviest elements represents today one of the most urgent and interesting problems of the natural sciences.





Similar content being viewed by others
Notes
In those years, Ya.I. Frenkel independently developed the theory of nuclear fission in the Soviet Union [5].
Vestnik Rossiiskoi Akademii Nauk (Journal of the Russian Academy of Sciences) with color images is published on https://sciencejournals.ru/journal/vestnik/ [in Russian]. The full texts of the journal are available without registration.
In 2019, the largest Periodic Table of the Chemical Elements (660 m2) is located at Edith Cowan University (ECU) in Perth, Australia.
Unfortunately, californium is the heaviest element that can be produced in a nuclear reactor in the amount needed to make a target. To synthesize the 119th element or heavier elements, it is necessary to increase the mass and charge of the bombarding ions.
REFERENCES
G. Gamov, “Discussion on the structure of atomic nuclei,” Proc. Royal Soc. A, No. 123, 386–387 (1929).
G. Gamov, “Zur Quantentheorie des Atomkernes,” Zeitschrift fur Physik 51 (3/4), 204–212 (1928).
C. F. Von Weizsä cker, “Zur Theorie der Kernmassen,” Zeitschrift fur Physik 96, 431 (1935).
N. Bohr and J. A. Weeler, “The mechanism of nuclear fission,” Phys. Rev. 56, 426–450 (1939).
Ya. I. Frenkel’, “Electrocapillary theory of the splitting of heavy nuclei by slow neutrons,” Zh. Eksp. Teoret. Fiz., No. 6, 641–653 (1939).
G. N. Flerov and K. A. Petrjak, “Spontaneous fission of uranium,” Phys. Rev. 58, 89 (1940).
G. T. Seaborg and W. D. Loveland, “Transuranium nuclei,” in Treatise on Heavy-Ion Science, Ed. by D. A. Brom-ley (Plenum Press, New York, 1985), Vol. 4, p. 289.
S. M. Polikanov, A. V. Druin, V. A. Karnaukhov, et al., “Spontaneous fission with an anomalously short period,” Soviet Physics JETP, No. 15, 1016–1021 (1962).
K. Morita, K. Morimoto, D. Kaji, et al., “Experiment on the synthesis of element 113 in the reaction 209Bi (70Zn,n)278113,” J. Phys. Soc. Jpn. 73 (10), 2593–2596 (2004).
Yu. Ts. Oganessian, V. K. Utyonkov, Yu. V. Lobanov, et al., “Synthesis of superheavy nuclei in the 48Ca + 244Pu reaction,” Phys. Rev. Lett. 83, 3154–3157 (1999).
Yu. Ts. Oganessian, V. K. Utyonkoy, Yu. V. Lobanov, et al., “Experiments on the synthesis of element 115 in the reaction 243Am(48Ca,xn)291−x115,” Phys. Rev. C 69 (021601(R)) (2004).
Yu. Ts. Oganessian, F. Sh. Abdullin, P. D. Bailey, et al., “Synthesis of a new element with atomic number Z = 117,” Phys. Rev. Lett. 104 (142502) (2010).
Yu. Ts. Oganessian, V. K. Utyonkov, Yu. V. Lobanov, et al., “Synthesis of the isotopes of elements 118 and 116 in the 249Cf and 245Cm + 48Ca fusion reactions,” Phys. Rev. C 74 (044602) (2006).
Yu. Ts. Oganessian and V. K. Utyonkov, “Super-heavy element research,” Rep. Prog. Phys. 78 (036301) (2015).
R. Eichler, N. V. Aksenov, A. V. Belozerov, et al., “Chemical characterization of element 112,” Nature 447, 72–75 (2007).
J.-M. Mewes, O. R. Smits, G. Kresse, et al., “Copernicium: A relativistic noble liquid,” Angew. Chem., Int. Ed. Engl. 58, 17964–17968 (2019).
W. Ramsay and F. Soddy, “Further experiments on the production of helium from radium,” Proc. Royal Soc. (1854–1905) 73, 346–358 (1904).
V. Pershina, “Relativity in the electronic structure of the heaviest elements and its influence on periodicities in properties,” Radiochimica Acta 107, 833–864 (2019).
E. Eliav, A. Borschevsky, and U. Kaldor, “Electronic structure at the edge of the Periodic Table,” Nucl. Phys. News 29, 16–20 (2019).
B. G. C. Lackenby, V. A. Dzuba, and V. V. Flambaum, “Atomic structure calculations of superheavy noble element oganesson (Z = 118),” Phys. Rev. A 98, (042512) (2018).
P. Jerabek, B. Schuetrumpf, P. Schwerdtfeger, and W. Nazarewicz, “Electron and nucleon localization functions of oganesson: Approaching the Thomas–Fermi limit,” Phys. Rev. Lett. 120, (053001) (2018).
V. M. Shabaev, I. I. Tupitsyn, M. Y. Kaygorodov, and Y. S. Kozhedub, Localization of electron states of Oganesson atoms. Paper presented at the 4th International Symposium on Superheavy Elements (SHE2019), Hakone, Japan, Dec. 1–5, 2019.
S. A. Giuliani, Z. Matheson, W. Nazarewicz, et al., “Colloquium: Superheavy elements: Oganesson and beyond,” Rev. Mod. Phys. 91 (1), (01100) (2019).
C. E. Düllmann, “Superheavy element research at TASCA at GSI,” Fission Properties Neutron-Rich Nuclei 44, 271–277 (2013).
S. Hofmann, S. Heinz, R. Mann, et al., “Review of even element super-heavy nuclei and search for element 120,” Eur. Phys. J. A 52, 180 (2016).
Yu. Ts. Oganessian, V. K. Utyonkov, Yu. V. Lobanov, et al., “Attempt to produce element 120 in the 244Pu + 58Fe reaction,” Phys. Rev. C 79, (024603) (2009).
A. Borschevsky, V. Pershina, E. Eliav, and U. Kaldor, “Ab initio predictions of atomic properties of element 120 and its lighter group-2 homologues,” Phys. Rev. A 87, (022502) (2013).
G. G. Gulbekian, S. N. Dmitriev, M. G. Itkis, et al., “Start-up of the DC-280 cyclotron, the basic facility of the factory of superheavy elements of the Laboratory of Nuclear Reactions at the Joint Institute for Nuclear Research,” Phys. Part. Nucl. Lett. 16 (6), 866–875 (2019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
I consider it a pleasant duty to thank Academician A.P. Khokhlov for the invitation to speak at the General Meeting of the Members of the Russian Academy of Sciences with this report and E.V. Chernyshev for his help in preparing the article.
Additional information
Translated by O. Zhukova
RAS Academician Yuri Tsolakovich Oganessian is the Scientific Director of the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research.
Rights and permissions
About this article
Cite this article
Oganessian, Y.T. 150th Anniversary of the Periodic Table of the Chemical Elements. Her. Russ. Acad. Sci. 90, 207–213 (2020). https://doi.org/10.1134/S1019331620020148
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1019331620020148