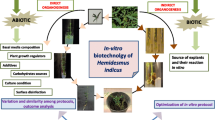
Abstract
Hemidesmus indicus (L.) R. Br. ex Schult is commonly known as anantmul or Indian sarsaparilla. The roots of this plant, which display a wide range of medicinal, biological, and phytopharmaceutical properties, are used in the pharmaceutical and food industries. Conventionally, the plant is propagated by seed germination or vegetatively, but the efficacy of traditional methods has some limitations: plants derived from seed germination are prone to seed-borne diseases, or plantlet production using vegetative propagation is limited. In contrast, plant tissue culture allows for large-scale propagation and secondary metabolite production in vitro without sacrificing plants from their natural habitats. Many efforts have been made over 40 years of research to establish efficient micropropagation protocols to speed up cultivation of this plant, including callus-mediated in vitro propagation, somatic embryogenesis, and shoot multiplication using cotyledenory nodes, stem segments, shoot tips, and nodal explants. Among these explants, nodal explants are the most commonly used for H. indicus micropropagation. The application of adenine sulfate, citric acid, ascorbic acid, and arginine may be useful in preventing explant browning, premature leaf senescence, and shoot tip abscission during in vitro culture. This review provides insight into micropropagation, use of synthetic seeds for short-term germplasm preservation, and in vitro production of secondary metabolites such as 2-hydroxy-4-methoxybenzaldehyde, lupeol, vanillin, and rutin, from in vitro root and callus cultures. Furthermore, unexplored and possible innovative areas of research in Hemidesmus biotechnology are also discussed.
Key points
• Hemidesmus indicus has multiple therapeutic applications.
• H. indicus roots are used in confectionary and pharmacy.
• This review comprehensively assesses H. indicus tissue culture.
• Challenges and future research of H. indicus biotechnology are discussed.

Similar content being viewed by others
Change history
22 August 2020
Following publication of the original article (Kher et al. 2020), the authors identified following mistake in the author affiliation.
References
Batista DS, Felipe SHS, Silva TD, de Castro KM, Mamedes-Rodrigues TC, Miranda NA, Ríos-Ríos AM, Faria DV, Fortini EA, Chagas K, Torres-Silva G, Xavier A, Arencibia AD, Otoni WC (2018) Light quality in plant tissue culture: does it matter? In Vitro. Cell Dev Biol Plant 54:195–215. https://doi.org/10.1007/s11627-018-9902-5
Benerjee A, Ganguly S (2014) Medicinal importance of Hemidesmus indicus: a review on its utilities from ancient Ayurveda to 20th century. Adv Biores 5:208–213. https://doi.org/10.15515/abr.0976
Beyl CA (2011) Getting started with tissue culture: media preparation, sterile technique, and laboratory equipment. In: Trigiano RN, Gray DJ (eds) Plant tissue culture, development, and biotechnology. CRC Press, Inc., Boca Raton, Florida, USA, pp 11–26. https://doi.org/10.1201/F9780203506561-7
Bi WL, Pan C, Hao XY, Cui ZH, Kher MM, Marković Z, Wang QC, Teixeira da Silva JA (2017) Cryopreservation of grapevine (Vitis spp.)—a review. In Vitro Cell Dev Biol Plant 53:449–460. https://doi.org/10.1007/s11627-017-9822-9
Chakraborty D, Sircar D, Mitra A (2008) Phenylalanine ammonia-lyase-mediated biosynthesis of 2-hydroxy-4-methoxybenzaldehyde in roots of Hemidesmus indicus. J Plant Physiol 165:1033–1040. https://doi.org/10.1016/j.jplph.2007.09.002
Chang S, Mahon EL, MacKay HA, Rottmann WH, Strauss SH, Pijut PM, Powell WA, Coffey V, Lu H, Mansfield SD, Jones TJ (2018) Genetic engineering of trees: progress and new horizons. In Vitro Cell Dev Biol Plant 54:341–376. https://doi.org/10.1007/s11627-018-9914-1
Cheruvathur MK, Najeeb N, Thomas TD (2013) In vitro propagation and conservation of Indian sarsaparilla, Hemidesmus indicus L. R. Br. through somatic embryogenesis and synthetic seed production. Acta Physiol Plant 35:771–779. https://doi.org/10.1007/s11738-012-1117-5
Devi B, Mohan C, Manjula P, Kiran KB, Naresh B, Prathibha DB (2014) Phytochemical and micropropagation studies in Hemidesmus indicus (L.) R. Br. J Indian Bot Soc 93:76–81
Efloras (2020) Hemidesmus indicus (L.) R. Br. http://www.efloras.org/florataxon.aspx?flora_id=5&taxon_id=115013. Accessed: 23 May, 2020
Espinosa-Leal CA, Puente-Garza CA, García-Lara S (2018) In vitro plant tissue culture: means for production of biological active compounds. Planta 248:1–18. https://doi.org/10.1007/s00425-018-2910-1
Faisal M, Alatar A (2019) Synthetic seeds: germplasm regeneration, preservation and prospects. Springer International Publishing, Cham. https://doi.org/10.1007/978-3-030-24631-0
Fiori J, Leoni A, Fimognari C, Turrini E, Hrelia P, Mandrone M, Iannello C, Antognoni F, Poli F, Gotti R (2014) Determination of phytomarkers in pharmaceutical preparations of Hemidesmus indicus roots by micellar electrokinetic chromatography and high-performance liquid chromatography–mass spectrometry. Anal Lett 47:2629–2642. https://doi.org/10.1080/00032719.2014.917423
FRLGHT (2020) Hemidesmus indicus (L.) Schult. In: FRLGHT, Indian Med. Plant Database. http://www.medicinalplants.in/searchpage/showdetails/xplant_id/e2d232e3dcd00751bdf511c1ac0d0195. Accessed: 23 May, 2020
Gamborg OL, Miller RAA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158. https://doi.org/10.1016/0014-4827(68)90403-5
George S (2009) PhD thesis “In vitro propagation, conservation and phytochemical studies of Decalepis habflltomi Wight & Arn., Hemidesmus indicus (L.) R. Br. and Utleria salicifolia Bedd. ex Hook. f.” Department of Biotechnology and Microbiology, Kannur University, Kerala, India, pp 1–189
Ghatge SR (2007) In vitro culture studies in medicinal plants viz. Hemidesmus indicus (L.) Schult and Rubia cordifolia L. PhD thesis, Department of Botany, Shivaji University, Kolhapur, Maharastra, India, pp 1–162
Ghatge S, Dixit GB (2006) Somatic embryogenesis and plant regeneration from leaf cultures of Hemidesmus indicus R. Br. a medicinal plant. In: International Symposium on Frontiers in Genetics and Biotechnology-Retrospect and Prospects, p 179
Ghatge SR, Kedage VV, Dixit GB (2008) Somatic embryogenesis and plant regeneration of a rare and multipurpose medicinal plant Hemidesmus indicus (L.) Schult. Plant Cell Biotechnol Mol Biol 9:71–74
Gopi S (2014) M.Sc. Thesis on “Induction and stablishmnt of transformed hairy root culturs of Sarsaparilla (Hemidesmus indicus L.) R. Br.” Departmnt of Plant Biotchnology, Faculty of Agriculture, Kerala Agriculture University, pp 1–78
Heble MR, Chadha MS (1978) Steroids in cultured tissues and mature plant of Hemidesmus indicus RBr. (Asclepiadiaceae). Zeit Pflanzenphysiol 89:401–406. https://doi.org/10.1016/S0044-328X(78)80036-1
Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K (2016) Plant regeneration: cellular origins and molecular mechanisms. Development 143:1442–1451. https://doi.org/10.1242/dev.134668
Isah T, Umar S, Mujib A, Sharma MP, Rajasekharan PE, Zafar N, Frukh A (2018) Secondary metabolism of pharmaceuticals in the plant in vitro cultures: strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult 132:239–265. https://doi.org/10.1007/s11240-017-1332-2
Khan PA (2014) Tissue culture studies in Hemidesmus indicus (L.) R. Br. Golden Res Thoughts 4:1–6
Krishna H, Alizadeh M, Singh D, Singh U, Chauhan N, Eftekhari M, Sadh RK (2016) Somaclonal variations and their applications in horticultural crops improvement. 3. Biotech 6:1–18. https://doi.org/10.1007/s13205-016-0389-7
Kulus D (2019) Managing plant genetic resources using low and ultra-low temperature storage: a case study of tomato. Biodivers Conserv 28:1003–1027. https://doi.org/10.1007/s10531-019-01710-1
Kulus D, Zalewska M (2014) Cryopreservation as a tool used in long-term storage of ornamental species – a review. Sci Hortic 168:88–107. https://doi.org/10.1016/j.scienta.2014.01.014
Kundu A, Jawali N, Mitra A (2012) Shikimate pathway modulates the elicitor-stimulated accumulation of fragrant 2-hydroxy-4-methoxybenzaldehyde in Hemidesmus indicus roots. Plant Physiol Biochem 56:104–108. https://doi.org/10.1016/j.plaphy.2012.04.005
Lin ML, Staba EJ (1961) Peppermint and spearmint tissue cultures. I. Callus formation and submerged culture. Lloydia 24:139–145
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int Plant Propagators’ Soc Proc 30:421–427
Maity SK (2018a) A micropropagation technique of Hemidesmus indicus (Asclepiadaceae), a valuable medicinal plant. Flora Fauna 24:16–20
Maity SK (2018b) Rapid and large scale micropropagation technique of Hemidesmus indicus R. Br. (Asclepiadaceae) which is a valuable medicinal plant all over India. In: De D, Roy S, Bera GC (eds) Biotechnology and Nature. Santi Mudran, 32/3 Patuatola Lane, Kolkata-700009, pp 177–180
Malathy S, Pai JS (1998) In vitro propagation of Hemidesmus indicus. Fitoterapia 69:533–536
Miler N, Kulus D, Woźny A, Rymarz D, Hajzer M, Wierzbowski K, Nelke R, Szeffs L (2019) Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: a study on plant quality and cost reduction. In Vitro Cell Dev Biol Plant 55:99–108. https://doi.org/10.1007/s11627-018-9939-5
Misra N, Mehrotra S (2006) Effect of mutagens on production of secondary metabolites in callus culture of Indian Sarsaparilla (Hemidesmus indicus). Hortic Environ Biotechnol 47:23–27. https://doi.org/10.3923/jps.2008.146.156
Misra N, Misra P, Datta SK, Mehrotra S (2003) Improvement in clonal propagation of Hemidesmus indicus R. Br. through adenine sulphate. J Plant Biotechnol 5:239–244
Misra N, Misra P, Datta SK, Mehrotra S (2005) In vitro biosynthesis of antioxidants from Hemidesmus indicus R. Br. cultures. In Vitro Cell Dev Biol Plant 41:285–290. https://doi.org/10.1079/ivp2004627
Mitra M, Gantait S, Mandal N (2020) Coleus forskohlii: advancements and prospects of in vitro biotechnology. Appl Microbiol Biotechnol. (in press). https://doi.org/10.1007/s00253-020-10377-6
Mukherjee E, Gantait S, Kundu S, Sarkar S, Bhattacharyya S (2019) Biotechnological interventions on the genus Rauvolfia: recent trends and imminent prospects. Appl Microbiol Biotechnol 103:7325–7354. https://doi.org/10.1007/s00253-019-10035-6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nagahatenna DSK, Peiris SE (2007) In vitro propagation of Hemidesmus indicus (L.) R . Br . (Iramusu) through nodal culture. Trop Agric Res 19:181–192
Nagahatenna DSK, Peiris SE (2008) Modification of plant architecture of Hemidesmus indicus (L.) R . Br . (Iramusu) by In vitro colchicine treatment. Trop Agric Res 20:234–242
Nandy S, Mukherjee A, Pandey DK, Ray P, Dey A (2020) Indian sarsaparilla (Hemidesmus indicus): recent progress in research on ethnobotany, phytochemistry and pharmacology. J Ethnopharmacol (in press) 112609. https://doi.org/10.1016/j.jep.2020.112609
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163(3862):85–87. https://doi.org/10.1126/science.163.3862.85
NMPB (2020) Hemidesmus indicus (L.) R.Br. ex Schult. In: National Medicinal Plants Board, Ministry of AYUSH, Government of India. https://nmpb.nic.in/medicinal_list. Accessed: 23 May, 2020
Pathak AR, Joshi AG (2017) Indirect organogenesis from leaf explants of Hemidesmus indicus (L.) R. Br.: an important medicinal plant. Plant Biosyst 151:1–5. https://doi.org/10.1080/11263504.2015.1108938
Pathak AR, Joshi AG, Shrivastava N, Sharma P (2017) Regeneration and chemical profiling in Hemidesmus indicus (L.) R. Br. South African J Bot 113:413–420. https://doi.org/10.1016/j.sajb.2017.09.022
Patidar N (2017) Callusing and organogenesis in Shodhganga or Anantmoola (Hemidesmus indicus Linn.). M.Sc. Thesis, Departement of Plant Breeding and genetics, Rajmata Vijayaraje Scindia Krishi Vishwa Vidyalaya, Gwalior, pp 1–51
Patnaik J, Debata BK (1996) Micropropagation of Hemidesmus indicus (L.) R. Br. through axillary bud culture. Plant Cell Rep. 15:427–430
Pospíšilová J, Tichá I, Kadlechek P, Haisel D, Plzáková Š (1999) Acclimatization of micropropagated plants to ex vitro conditions. Biol Plant 42:481–497. https://doi.org/10.1023/A:1002688208758
Prashanti M, Kalpana K, Shivaprasad P, Singh S, Rojarani A, Reddy KJ (2017) Rapid in vitro propagation of medicinally important Hemidesmus indicus (L.) R. BR. through nodal explants. J Indian Bot Soc 96:76–81
Purohit P, Bais RT, Singh P, Khan S (2014) In vitro regeneration of Hemidesmus indicus L. R. Br an important endangered medicinal plant. UK J Pharm Biosci 2:25–31
Qahtan AA, Abdel-Salam EM, Alatar AA, Wang Q-C, Faisal M (2019) An introduction to synthetic seeds: production, techniques, and applications. In: Faisal M, Alatar AA (eds) Synthetic seeds: germplasm regeneration, preservation and prospects. Springer International Publishing, Cham, pp 1–20. https://doi.org/10.1007/978-3-030-24631-0_1
Raghuramulu D, Murthy KSR, Pullaiah T (2003) Regeneration of plants from root segments derived from aseptic seedlings of Hemidesmus indicus R.Br. Phytomorphology 53:293–298
Raghuramulu D, Murthy KSR, Pullaiah T (2005) Vegetative propagation of Hemidesmus indicus R.Br. by stem cuttings. Indian For 131:1505–1508
Reddy GS, Reddy YM, Saritha KV (2016) Effect of plant growth regulators on in vitro propagation of Hemidesmus indicus (L.) R. Br.- an important aromatic medicinal herb. Sci Spectr 1:366–370
Saha S, Mukhopadhyay MJ, Mukhopadhyay S (2003) In vitro clonal propagation through bud culture of Hemidesmus indicus (L) R Br: an important medicinal herb. J Plant Biochem Biotechnol 12:61–64. https://doi.org/10.1007/BF03263162
Sandhu M, Wani SH, Jiménez VM (2018) In vitro propagation of bamboo species through axillary shoot proliferation: a review. Plant Cell Tissue Organ Cult 132:27–53. https://doi.org/10.1007/s11240-017-1325-1
Sarasan V, Nair GM (1991) Tissue culture of medicinal plants: morphogenesis, direct regeneration and somatic embryogenesis. In: Prakash J, Pierik RLM (eds) Horticulture — new technologies and applications. Current Plant Science and Biotechnology in Agriculture, vol 12. Springer, Dordrecht, pp 237–240
Sarsan V, Soniya EV, Nair GM (1994) Regeneration of Indian sarsaparilla, Hemidesmus indicus R.Br., through organogenesis and somatic embryogenesis. Indian J Exp Biol 32:284–287
Saryam R, Seniya C, Khan S (2012a) In-vitro micropropagation of Hemidesmus indicus an important medicinal plant. Int J Compr Pharm 3:1–3
Saryam R, Seniya C, Khan S (2012b) In-vitro micropropagation of Hemidesmus indicus an important medicinal plant. J Pharm Res 5:5467–5469
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204. https://doi.org/10.1139/b72-026
Shanmugapriya AK, Sivakumar T (2011) Regeneration of in-vitro plantlets in Hemidesmus indicus (L.) R. Br. through nodal and leaf explants. Int Multidiciplinary Res J 1:41–45
Sharma PC, Yelne MB (1995) Observations on in vitro propagation of Sariva (Hemidesmus indicus R. Br). Bull Medico-Ethano-Botanical Res 16:129–132
Sharma S, Shahzad A, Teixeira da Silva JA (2013) Synseed technology—a complete synthesis. Biotechnol Adv 31:186–207. https://doi.org/10.1016/j.biotechadv.2012.09.007
Shekhawat MS, Manokari M (2016) In vitro regeneration frequency, micro-morphological studies and ex vitro rooting of Hemidesmus indicus (L.) R. Br.: a multi-potent endangered climber. Indian J Plant Physiol 21:151–160. https://doi.org/10.1007/s40502-016-0216-5
Siddique NA, Bari MA (2006) Plant regeneration from axillary shoot segments derived callus in Hemidesmus indicus (L.) R. Br. (Anantamul) an endangered medicinal plant in Bangladesh. J Plant Sci 5:61–67
Siddique NA, Bari MA (2010) Plant regeneration from axillary shoot segments derived callus in Hemidesmus indicus (L.) R. Br. (Anantamul) an endangered medicinal plant in Bangladesh. J Plant Sci 1:42–48
Siddique NA, Bari MA, Khatun N, Rahman MH, Rahman MH, Huda S (2003) Plant regeneration from nodal segments derived callus in Hemidesmus indicus (L.) R. Br (Anantamul) an endangered medicinal plant in Bangladesh. J Biol Sci 3:1158–1163. https://doi.org/10.3923/jbs.2003.1158.1163
Sindura KP (2014) Establishment of in vitro root cultures of sarsaparilla (Hemidesmus indicus L.) R. Br. M.Sc. Thesis, Departmnt of Plant Biotchnology, Faculty of Agriculture, Kerala Agriculture University, pp 1–73
Singh K, Shalini AK (2015) Micropropagation and multiple shoot formation from nodal explants of Hemidesmus indicus (L.) R. Br. - a rare medicinal plant. Intrnational J Adv Res 3:725–728
Sood P, Singh RK, Prasad M (2019) Millets genetic engineering: the progress made and prospects for the future. Plant Cell Tissue Organ Cult 137:421–439. https://doi.org/10.1007/s11240-019-01587-6
Sreekumar S (1997) In vitro culture and secondary metabolite production in Hemidesmus indicus R . Br . PhD Thesis, Plant Biotechnology Division, Tropical Botanical Garden and Research Institute, Paldode, Thiruvanthapuram, Kerala, India, pp 1–180
Sreekumar S, Seeni S, Pushpangadan P (1998) Production of 2-hydroxy 4-methoxy benzaldehyde using root cultures of Hemidesmus indicus. Biotechnol Lett 20:631–635. https://doi.org/10.1023/A:1005354003727
Sreekumar S, Seeni S, Pushpangadan P (2000) Micropropagation of Hemidesmus indicus for cultivation and production of 2-hydroxy 4-methoxy benzaldehyde. Plant Cell Tissue Organ Cult 62:211–218. https://doi.org/10.1023/A:1006486817203
Sreelekha A, Jirovetz L, Shafi PM (2007) Comparative study of the essential oils from Hemidesmus indicus and Decalepis hamiltonii. Asian J Chem 19:4942–4944
Sudarmani P, Hasina M (2013) In vitro regeneration and sodium chloride salt tolerant Hemidesmus indicus (L.) R.Br. plant production. ANJAC Journal of Science 12:55–62
Suryavanshi S, Choudhari A, Raina P, Kaul-Ghanekar R (2019) A polyherbal formulation, HC9 regulated cell growth and expression of cell cycle and chromatin modulatory proteins in breast cancer cell lines. J Ethnopharmacol 242:112022. https://doi.org/10.1016/j.jep.2019.112022
Teixeira da Silva JA (2012a) Is BA (6-benzyladenine) BAP (6-benzylaminopurine)? Asian Aust J Plant Sci Biotechnol 6 (special issue 1):121–124
Teixeira da Silva JA (2012b) Callus, calluses or calli: multiple plurals? Asian Aust J Plant Sci Biotechnol 6(special issue 1):125–126
Teixeira da Silva JA, Kulus D (2014) Chrysanthemum biotechnology: discoveries from the recent literature. Folia Hortic 26:67–77. https://doi.org/10.2478/fhort-2014-0007
Teixeira da Silva JA, Kulus D, Zhang X, Zeng S-J, Ma G-H, Piqueras A (2016) Disinfection of explants for saffron (Crocus sativus L.) tissue culture. Env Exp Biol 14:183–198. https://doi.org/10.22364/eeb.14.25
Teixeira da Silva JA, Zeng S, Godoy-Hernández G, Rivera-Madrid R, Dobránszki J (2019) Bixa orellana L. (achiote) tissue culture: a review. In Vitro Cell Dev Biol Plant 55:231–241. https://doi.org/10.1007/s11627-019-09969-3
The Plant List (2020) Hemidesmus indicus (L.) R. Br. ex Schult. http://www.theplantlist.org/tpl1.1/record/tro-2604022. Accessed: 23 May, 2020
Turrini E, Catanzaro E, Muraro MG, Governa V, Trella E, Mele V, Calcabrini C, Morroni F, Sita G, Hrelia P, Tacchini M, Fimognari C (2018) Hemidesmus indicus induces immunogenic death in human colorectal cancer cells. Oncotarget 9:24443–24456. doi: 10.18632/oncotarget.25325
Turrini E, Catanzaro E, Ferruzzi L, Guerrini A, Tacchini M, Sacchetti G, Paganetto G, Maffei F, Pellicioni V, Poli F, Hrelia P, Mandrone M, Sestili P, Brigotti M, Fimognari C (2019) Hemidesmus indicus induces apoptosis via proteasome inhibition and generation of reactive oxygen species. Sci Rep 9:7199. https://doi.org/10.1038/s41598-019-43609-5
Verma S, Vashistha BD (2016) In vitro multiplication of Indian sarsaparilla, Hemidesmus indicus (L.) R. Br. – an important medicinal herb through shoot tip explants. Int J Innov Res Sci Eng Technol 5:1542–1548. https://doi.org/10.15680/IJIRSET.2016.0502072
Wen SS, Chen L, Tian RN (2019) Micropropagation of tree peony (Paeonia sect. Moutan): a review. Plant Cell Tiss Organ Cult 141:1–14. https://doi.org/10.1007/s11240-019-01747-8
White PR (1934) Potentially unlimited growth of excised tomato root tips in a liquid medium. Plant Physiol 9:585–600. https://doi.org/10.1104/pp.9.3.585
Xiao Y, Niu G, Kozai T (2011) Development and application of photoautotrophic micropropagation plant system. Plant Cell Tissue Organ Cult 105:149–158. https://doi.org/10.1007/s11240-010-9863-9
Yadav V, Shahzad A, Ahmad Z, Sharma S, Parveen S (2019) Synthesis of nonembryonic synseeds in Hemidesmus indicus R. Br.: short term conservation, evaluation of phytochemicals and genetic fidelity of the regenerants. Plant Cell Tiss Organ Cult 138:363–376. https://doi.org/10.1007/s11240-019-01634-2
Yaseen M, Ahmad T, Sablok G, Standardi A, Hafiz IA (2013) Review: Role of carbon sources for in vitro plant growth and development. Mol Biol Rep 40:2837–2849. https://doi.org/10.1007/s11033-012-2299-z
Acknowledgments
The authors thank Professor T. Pullaiah (Department of Botany, Sir Krishnadevaraya University, Anantpur, AP, India), Dr. K. Sri Ramamurthy (Principal Scientist, Shivashakti Biotechnologies Ltd., Hyderabad, Telangana State, India), and Dr. Pratibha Mishra (Senior Principal Scientist, National Botanical Research Institute) for reprints.
Funding
Dr. Mahipal S. Shekhawat thanks the National Medicinal Plant Board, Ministry of AYUSH, Government of India (grant number NMPB/IFD/GIA/NR/PL/2018-19/187) for financial support.
Author information
Authors and Affiliations
Contributions
All authors have equal contribution in this review, and are responsible for the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kher, M.M., Shekhawat, M.S., Nataraj, M. et al. Indian sarsaparilla, Hemidesmus indicus (L.) R. Br. ex Schult: tissue culture studies. Appl Microbiol Biotechnol 104, 6463–6479 (2020). https://doi.org/10.1007/s00253-020-10714-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10714-9