Abstract
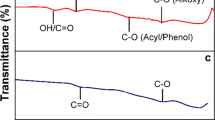
The present study investigates the structural and chemical factors contributing to the performance of graphene oxide anode materials produced by Hummers (GOH) and Tour (GOT) for lithium-ion batteries (LIBs). The GO synthesized by these methods were studied using FTIR and Raman spectroscopy, X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), and scanning electron microscopy (SEM). Thorough electrochemical analysis including cycling stability, rate capability, cyclic voltammetry (CV), and electrochemical impedance spectroscopy (EIS) were also conducted. We show that the improved performance of GOT (almost twice) compared with that of GOH is due to the key role of the protective agent (H3PO4) in reducing/inhibiting the hole formation during the synthesis of the GOT surface which in turn results in superior cycling performance over the GOH. The results and proposed mechanism presented in this work elucidates the role of structural factors and defects in preparing graphene-based anodes with enhanced electrochemical efficiency.

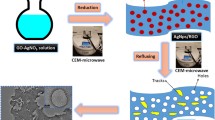
Graphical abstract













Similar content being viewed by others
References
Xiong D, Li X, Shan H, Zhao Y, Dong L, Xu H, Zhang X, Li D, Sun X (2015) Oxygen-containing functional groups enhancing electrochemical performance of porous reduced graphene oxide cathode in lithium ion batteries. Electrochim Acta 174:762–769. https://doi.org/10.1016/j.electacta.2015.06.041
Kim H, Park KY, Hong J, Kang K (2014) All-graphene-battery: bridging the gap between supercapacitors and lithium ion batteries. Sci Rep 4:5278. https://doi.org/10.1038/srep05278
Song Y, Hu Y, Sha Y, Rong H, Wen H, Liu HJ, Liu Q (2019) Graphene oxide linked with N, N′-diamino-1,4,5,8-naphthalenetetracarboxylic bisimide as a stable cathode material for lithium-ion batteries. Ionics (Kiel) 25:2987–2995. https://doi.org/10.1007/s11581-019-02868-y
Ershadi M, Javanbakht M et al (2018) A patent landscape on liquid electrolytes for lithium-ion batteries. Anal Bioanal Electrochem 10(12):1629–1653
Ershadi M, Javanbakht M, Mozaffari SA, Brandell D, Lee MT, Zahiri B (2020) Facile stitching of graphene oxide nanosheets with ethylenediamine as three dimensional anode material for lithium-ion battery. J Alloys Compd 818:152912. https://doi.org/10.1016/j.jallcom.2019.152912
Chen W, Hu Y, Lv W, Lei T, Wang X, Li Z, Zhang M, Huang J, du X, Yan Y, He W, Liu C, Liao M, Zhang W, Xiong J, Yan C (2019) Lithiophilic montmorillonite serves as lithium ion reservoir to facilitate uniform lithium deposition. Nat Commun 10:1–9. https://doi.org/10.1038/s41467-019-12952-6
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18:252–264. https://doi.org/10.1016/j.mattod.2014.10.040
Xia J, Liu L, Jamil S, Xie J, Yan H, Yuan Y, Zhang Y, Nie S, Pan J, Wang X, Cao G (2019) Free-standing SnS/C nanofiber anodes for ultralong cycle-life lithium-ion batteries and sodium-ion batteries. Energy Storage Mater 17:1–11. https://doi.org/10.1016/j.ensm.2018.08.005
Chen W, Lei T, Lv W, Hu Y, Yan Y, Jiao Y, He W, Li Z, Yan C, Xiong J (2018) Atomic interlamellar ion path in high sulfur content lithium-montmorillonite host enables high-rate and stable lithium–sulfur battery. Adv Mater 30:1–8. https://doi.org/10.1002/adma.201804084
Lei T, Chen W, Hu Y, Lv W, Lv X, Yan Y, Huang J, Jiao Y, Chu J, Yan C, Wu C, Li Q, He W, Xiong J (2018) A nonflammable and thermotolerant separator suppresses polysulfide dissolution for safe and long-cycle lithium-sulfur batteries. Adv Energy Mater 8:1–8. https://doi.org/10.1002/aenm.201802441
Lei T, Chen W, Huang J, Yan C, Sun H, Wang C, Zhang W, Li Y, Xiong J (2017) Multi-functional layered WS2 nanosheets for enhancing the performance of lithium–sulfur batteries. Adv Energy Mater 7:1–8. https://doi.org/10.1002/aenm.201601843
Lee DG, Yim T, Woo SG, Yu JS (2019) Amide-functionalized porous carbonaceous anode materials for lithium-ion batteries. ChemPhysChem 20(5):752–756. https://doi.org/10.1002/cphc.201801018
Wang H, Li X, Baker-Fales M, Amama PB (2016) 3D Graphene-based anode materials for Li-ion batteries. Curr Opin Chem Eng 13:124–132. https://doi.org/10.1016/j.coche.2016.08.009
Xu Y, Sheng K, Li C, Shi G (2010) Self-assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 4:4324–4330. https://doi.org/10.1021/nn101187z
An SJ, Li J, Daniel C, Mohanty D, Nagpure S, Wood DL III (2016) The state of understanding of the lithium-ion-battery graphite solid electrolyte interphase (SEI) and its relationship to formation cycling. Carbon N Y 105:52–76. https://doi.org/10.1016/j.carbon.2016.04.008
Vargas Ó, Caballero Á, Morales J (2015) Deficiencies of chemically reduced graphene as electrode in full Li-ion cells. Electrochim Acta 165:365–371. https://doi.org/10.1016/j.electacta.2015.03.039
Lian P, Zhu X, Liang S, Li Z, Yang W, Wang H (2010) Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim Acta 55:3909–3914. https://doi.org/10.1016/j.electacta.2010.02.025
Raccichini R, Varzi A, Wei D, Passerini S (2017) Critical insight into the relentless progression toward graphene and graphene-containing materials for lithium-ion battery anodes. Adv Mater 29:1–33. https://doi.org/10.1002/adma.201603421
Yuan G, Zhao X, Liang Y, Peng L, Dong H, Xiao Y, Hu C, Hu H, Liu Y, Zheng M (2019) Small nitrogen-doped carbon dots as efficient nanoenhancer for boosting the electrochemical performance of three-dimensional graphene. J Colloid Interface Sci 536:628–637. https://doi.org/10.1016/j.jcis.2018.10.096
Abdolhosseinzadeh S, Asgharzadeh H, Kim HS (2015) Fast and fully-scalable synthesis of reduced graphene oxide. Sci Rep 5:1–7. https://doi.org/10.1038/srep10160
Eigler S, Hirsch A (2014) Chemistry with graphene and graphene oxide - challenges for synthetic chemists. Angew Chem Int Ed 53:7720–7738. https://doi.org/10.1002/anie.201402780
Jiao D, Xie Z, Wan Q, Qu M (2019) Reduced irreversible capacities of graphene oxide-based anodes used for lithium ion batteries via alkali treatment. J Energy Chem 37:73–81. https://doi.org/10.1016/j.jechem.2018.11.018
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053. https://doi.org/10.1021/cr300115g
Georgakilas V, Tiwari JN, Kemp KC, Perman JA, Bourlinos AB, Kim KS, Zboril R (2016) Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem Rev 116:5464–5519. https://doi.org/10.1021/acs.chemrev.5b00620
Wang Y, Li X, He M, du H, Wu X, Hao J, Li B (2019) Core-shells on nanosheets: Fe3O4 @carbon-reduced graphene oxide composites for lithium-ion storage. J Solid State Electrochem 23:237–244. https://doi.org/10.1007/s10008-018-4105-x
Geng CY, Yu J, Shi FN (2019) Few-layers of graphene modified TiO2/graphene composites with excellent electrochemical properties for lithium-ion battery. Ionics (Kiel) 25:3059–3068. https://doi.org/10.1007/s11581-019-02894-w
Miao F, Cong W, Miao R, Wang N, Wu W, Zang Y, Shi C, Zhu L, Tao B, Chu PK (2019) High-performance anode materials based on 3D orderly and vertically macroporous graphene-Si framework for Li-ion batteries. Ionics (Kiel) 25:467–473. https://doi.org/10.1007/s11581-018-2653-9
Li W, Xu H, Cui M, Zhao J, Liu F, Liu T (2019) Synthesis of sulfonated graphene/carbon nanotubes/manganese dioxide composite with high electrochemical properties. Ionics (Kiel) 25:999–1006. https://doi.org/10.1007/s11581-018-2767-0
Ajdari FB, Kowsari E, Ehsani A (2018) Ternary nanocomposites of conductive polymer/functionalized GO/MOFs: synthesis, characterization and electrochemical performance as effective electrode materials in pseudocapacitors. J Solid State Chem 265:155–166. https://doi.org/10.1016/j.jssc.2018.05.038
Lei T, Chen W, Lv W, Huang J, Zhu J, Chu J, Yan C, Wu C, Yan Y, He W, Xiong J, Li Y, Yan C, Goodenough JB, Duan X (2018) Inhibiting polysulfide shuttling with a graphene composite separator for highly robust lithium-sulfur batteries. Joule 2:2091–2104. https://doi.org/10.1016/j.joule.2018.07.022
Zhao X, Zhang X, Li C, Sun X, Liu J, Wang K, Ma Y (2019) High-Performance lithium-ion capacitors based on CoO-graphene composite anode and holey carbon nanolayer cathode. ACS Sustain Chem Eng 7:11275–11283. https://doi.org/10.1021/acssuschemeng.9b00641
Li C, Zhang X, Sun C, Wang K, Sun X, Ma Y (2019) Recent progress of graphene-based materials in lithium-ion capacitors. J Phys D Appl Phys 52(14):143001. https://doi.org/10.1088/1361-6463/aaff3a
Zhang S, Li C, Zhang X, Sun X, Wang K, Ma Y (2017) High performance lithium-ion hybrid Capacitors employing Fe3O4-graphene composite anode and activated carbon cathode. ACS Appl Mater Interfaces 9:17136–17144. https://doi.org/10.1021/acsami.7b03452
Kumar R, Sahoo S, Joanni E, Singh RK, Tan WK, Kar KK, Matsuda A (2019) Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog Energy Combust Sci 75:100786. https://doi.org/10.1016/j.pecs.2019.100786
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339. https://doi.org/10.1021/ja01539a017
Alam SN, Sharma N, Kumar L (2017) Synthesis of graphene oxide (GO) by modified hummers method and its thermal reduction to obtain reduced graphene oxide (rGO). Graphene 6(1):1–8. https://doi.org/10.4236/graphene.2017.61001
Feicht P, Biskupek J, Gorelik TE, Renner J, Halbig CE, Maranska M, Puchtler F, Kaiser U, Eigler S (2019) Brodie’s or Hummers’ method: oxidation conditions determine the structure of graphene oxide. Chem - A Eur J 25:8955–8959. https://doi.org/10.1002/chem.201901499
Rodrigues AF, Newman L, Lozano N et al (2018) A blueprint for the synthesis and characterisation of thin graphene oxide with controlled lateral dimensions for biomedicine. 2D Mater 5(3):035020. https://doi.org/10.1088/2053-1583/aac05c
Poh HL, Šaněk F, Ambrosi A, Zhao G, Sofer Z, Pumera M (2012) Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 4:3515–3522. https://doi.org/10.1039/c2nr30490b
Marcano DC, Kosynkin DV, Berlin JM, Tour J et al (2010) Improved synthesis of graphene oxide. ACS Nano 4(8):4806–4814. https://doi.org/10.1021/nn1006368
Li J, Zeng X, Ren T, van der Heide E (2014) The preparation of graphene oxide and its derivatives and their application in bio-tribological systems. Lubricants 2:137–161. https://doi.org/10.3390/lubricants2030137
Higginbotham AL, Kosynkin DV, Sinitskii A, Sun Z, Tour JM (2010) Lower-defect graphene oxide nanotubes. ACS Nano 4(4):2059–2069. https://doi.org/10.1021/nn100118m
Beydaghi H, Javanbakht M, Kowsari E (2014) Synthesis and characterization of poly(vinyl alcohol)/Sulfonated graphene oxide nanocomposite membranes for use in proton exchange membrane fuel cells (PEMFCs). Ind Eng Chem Res 53:16621–16632. https://doi.org/10.1021/ie502491d
Dezfuli AS, Ganjali MR, Naderi HR, Norouzi P (2015) A high performance supercapacitor based on a ceria/graphene nanocomposite synthesized by a facile sonochemical method. RSC Adv 5:46050–46058. https://doi.org/10.1039/c5ra02957k
Moo JGS, Khezri B, Webster RD, Pumera M (2014) Graphene oxides prepared by Hummers’, Hofmann’s, and Staudenmaier’s methods: dramatic influences on heavy-metal-ion adsorption. ChemPhysChem 15:2922–2929. https://doi.org/10.1002/cphc.201402279
Konios D, Stylianakis MM, Stratakis E, Kymakis E (2014) Dispersion behaviour of graphene oxide and reduced graphene oxide. J Colloid Interface Sci 430:108–112. https://doi.org/10.1016/j.jcis.2014.05.033
Guerrero-Contreras J, Caballero-Briones F (2015) Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater Chem Phys 153:209–220. https://doi.org/10.1016/j.matchemphys.2015.01.005
Huang HH, De Silva KKH, Kumara GRA, Yoshimura M (2018) Structural evolution of hydrothermally derived reduced graphene oxide. Sci Rep 8:2–10. https://doi.org/10.1038/s41598-018-25194-1
Albers RF, Bini RA, Souza JB et al (2019) A general one-pot synthetic strategy to reduced graphene oxide (rGO) and rGO-nanoparticle hybrid materials. Carbon N Y 143:73–84. https://doi.org/10.1016/j.carbon.2018.10.087
Kim NH, Kuila T, Lee JH (2013) Simultaneous reduction, functionalization and stitching of graphene oxide with ethylenediamine for composites application. J Mater Chem A 1:1349–1358. https://doi.org/10.1039/c2ta00853j
Du M, Sun J, Chang J et al (2014) Synthesis of nitrogen-doped reduced graphene oxide directly from nitrogen-doped graphene oxide as a high-performance lithium ion battery anode. RSC Adv 4:42412–42417. https://doi.org/10.1039/c4ra05544f
Yu H, Zhang B, Bulin C, Li R, Xing R (2016) High-efficient synthesis of graphene oxide based on improved Hummers method. Sci Rep 6:1–7. https://doi.org/10.1038/srep36143
Li C, Zhang X, Wang K, Sun X, Ma Y (2018) High-power and long-life lithium-ion capacitors constructed from N-doped hierarchical carbon nanolayer cathode and mesoporous graphene anode. Carbon N Y 140:237–248. https://doi.org/10.1016/j.carbon.2018.08.044
Zhang C, Mahmood N, Yin H, Liu F, Hou Y (2013) Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv Mater 25:4932–4937. https://doi.org/10.1002/adma.201301870
Sheng X, Cai W, Zhong L, Xie D, Zhang X (2016) Synthesis of functionalized graphene/polyaniline nanocomposites with effective synergistic reinforcement on anticorrosion. Ind Eng Chem Res 55:8576–8585. https://doi.org/10.1021/acs.iecr.6b01975
Kang JH, Chen JS (2018) Using ethylenediamine to prepare three dimensional nitrogen-doped graphene aerogel/sulfur composite for lithium-sulfur batteries. Diam Relat Mater 88:222–229. https://doi.org/10.1016/j.diamond.2018.07.015
Lee W, Lee JU, Jung BM, Byun JH, Yi JW, Lee SB, Kim BS (2013) Simultaneous enhancement of mechanical , electrical and thermal properties of graphene oxide paper by embedding dopamine. Carbon N Y 65:296–304. https://doi.org/10.1016/j.carbon.2013.08.029
Zhao C, Gao H, Chen C, Wu H (2015) Reduction of graphene oxide in Li-ion batteries. J Mater Chem A 3:18360–18364. https://doi.org/10.1039/c5ta05068e
Wu ZL, Liu F, Li CK, Chen XQ, Yu JG (2016) A sandwich-structured graphene-based composite: Preparation, characterization, and its adsorption behaviors for Congo red. Colloids Surf A Physicochem Eng Asp 509:65–72. https://doi.org/10.1016/j.colsurfa.2016.08.084
Mungse HP, Singh R, Sugimura H, Kumar N, Khatri OP (2015) Molecular pillar supported graphene oxide framework: conformational heterogeneity and tunable d-spacing. Phys Chem Chem Phys 17:20822–20829. https://doi.org/10.1039/C5CP02313K
Pham HD, Pham VH, Cuong TV (2011) Synthesis of the chemically converted graphene xerogel with superior electrical conductivity. ChemComm 47(34):9672–9674. https://doi.org/10.1039/c1cc13329b
Ossonon BD, Daniel B (2017) Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv 7(44):27224–27234. https://doi.org/10.1039/C6RA28311J
Zhang J, Cao H, Tang X, Fan W, Peng G, Qu M (2013) Graphite/graphene oxide composite as high capacity and binder-free anode material for lithium ion batteries. J Power Sources 241:619–626. https://doi.org/10.1016/j.jpowsour.2013.05.001
Stournara ME, Shenoy VB (2011) Enhanced Li capacity at high lithiation potentials in graphene oxide. J Power Sources 196:5697–5703. https://doi.org/10.1016/j.jpowsour.2011.02.024
Kim TH, Jeon EK, Ko Y, Jang BY, Kim BS, Song HK (2014) Enlarging the d-spacing of graphite and polarizing its surface charge for driving lithium ions fast. J Mater Chem A 2:7600–7605. https://doi.org/10.1039/c3ta15360f
Jang BZ, Liu C, Neff D, Yu Z, Wang MC, Xiong W, Zhamu A (2011) Graphene surface-enabled lithium ion-exchanging cells: Next-generation high-power energy storage devices. Nano Lett 11:3785–3791. https://doi.org/10.1021/nl2018492
Xu Y, Lin Z, Zhong X, Papandrea B, Huang Y, Duan X (2015) Solvated graphene frameworks as high-performance anodes for lithium-ion batteries. Angew Chem Int Ed 54:5345–5350. https://doi.org/10.1002/anie.201500677
Bulusheva LG, Stolyarova SG, Chuvilin AL, Shubin YV, Asanov IP, Sorokin AM, Mel’gunov MS, Zhang S, Dong Y, Chen X, Song H, Okotrub AV (2018) Creation of nanosized holes in graphene planes for improvement of rate capability of lithium-ion batteries. Nanotechnology 29(13):134001. https://doi.org/10.1088/1361-6528/aaa99f
Xiong D, Li X, Shan H, Yan B, Li D, Langford C, Sun X (2016) Scalable synthesis of functionalized graphene as cathodes in Li-ion electrochemical energy storage devices. Appl Energy 175:512–521. https://doi.org/10.1016/j.apenergy.2016.03.105
Song X, Chen Q, Shen E, Liu H (2019) Supercapacitive performances of few-layer MoS 2 on reduced graphene oxides. J Solid State Electrochem 23:911–923. https://doi.org/10.1007/s10008-019-04195-8
Qin F, Zhang K, Zhang Z, Fang J, Li J, Lai Y, Huang H (2019) Graphene/carbon aerogel for high areal capacity sulfur cathode of Li-S batteries. Ionics 25(10):4615–4624. https://doi.org/10.1007/s11581-019-03046-w
Yu P (1999) Determination of the lithium ion diffusion coefficient in graphite. J Electrochem Soc 146:8–14. https://doi.org/10.1149/1.1391556
Li S, Wang B, Liu J, Yu M (2014) In situ one-step synthesis of CoFe2O4/graphene nanocomposites as high-performance anode for lithium-ion batteries. Electrochim Acta 129:33–39. https://doi.org/10.1016/j.electacta.2014.02.039
Chen P, Zheng G, Guo G, Wang Z, Tang J, Li S, Wen Z, Ji S, Sun J (2019) Ce-doped V2O5 microspheres with improved electrochemical performance for high-power rechargeable lithium ion batteries. J Alloys Compd 784:574–583. https://doi.org/10.1016/j.jallcom.2018.12.373
Xiang HF, Li ZD, Xie K, Jiang JZ, Chen JJ, Lian PC, Wu JS, Yu Y, Wang HH (2012) Graphene sheets as anode materials for Li-ion batteries: Preparation, structure, electrochemical properties and mechanism for lithium storage. RSC Adv 2:6792–6799. https://doi.org/10.1039/c2ra20549a
Raccichini R, Varzi A, Passerini S, Scrosati B (2015) The role of graphene for electrochemical energy storage. Nat Mater 14:271–279. https://doi.org/10.1038/nmat4170
Acknowledgment
The authors are grateful to Amirkabir University of Technology (Tehran, Iran) and the Renewable Energy Research Center (RERC) for the technical support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
- The role of protective agent in preventing or reducing holes on graphene oxide surface was investigated.
- GOT improved about twice the reversible charging capacity comparing with GOH at different scan rates.
- GOT showed coulombic efficiency and charge capacity retention of ~92% and ~87% comparing with GOH sample (~86% and ~78%), respectively.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ershadi, M., Javanbakht, M., Mozaffari, S.A. et al. Preparing graphene-based anodes with enhanced electrochemical performance for lithium-ion batteries. Ionics 26, 4877–4895 (2020). https://doi.org/10.1007/s11581-020-03632-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-020-03632-3