Abstract
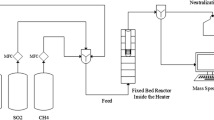
It has been shown in our previous findings that Mo-, W- and Re-based catalysts promoted with Ni, Co, and K are considered as a promising tri-component catalyst for sour WGS reaction. In order to evaluate the stability of the formed active sulfide and oxysulfidic species at sulfided Mo-, W- and Re-based catalysts at the same surface density of the main component, namely 2 atoms metal/nm2 γ-Al2O3 support, under influence of oxidizing agents as H2O steam and CO2, the WGS reaction was proceeded in absence of sulfur in gas feed. With the intent to enable a quick check, the applied approach was to perform the WGS reaction at high reaction temperature, 400 °C, in H2S-free syngas and varying the gas hourly space velocity (GHSV). This study gave information about the behaviour of sour molybdenum, tungsten and rhenium WGS catalysts during operation accident. It was found that the active sulfide and oxysulfidic species are more stable in absence of sulfur in feed at tri-component KCoMo and KCoRe catalysts. These catalysts possess lowest sulfur dependence of WGS activity and stability of the deactivation, which is an indication of the applicability of these two systems as optimal sour water–gas shift catalysts.



Similar content being viewed by others
References
Reddy GK, Smirniotis PG (2015) WGS reaction over Co-Mo sulfided catalysts. Water gas shift reaction: research developments and applications. Elsevier, Amsterdam, pp 101–126
Tonkovich A, Zilka J, La Mont M, Wang Y, Wegeng R (1999) Microchannel reactors for fuel processing applications. I. Water gas shift reactor. Chem Eng Sci 54:2947–2951
Song Ch (2002) Fuel processing for low-temperature and high-temperature fuel cells: challenges, and opportunities for sustainable development in the 21st century. Catal Today 77:17–49
Ruettinger W, Ilinich O, Farrauto RJ (2003) A new generation of water gas shift catalysts for fuel cell applications. J Power Sources 118:61–65
Stiegel GJ, Ramezan M (2006) Hydrogen from coal gasification: an economical pathway to a sustainable energy future. Int J Coal Geol 65:173–190
Ladebeck JR, Wagner J P (2003) Catalyst development for water-gas shift. In: Vielstich W, Lamm A, Gasteiger HA (eds) Handbook of fuel cells—fundamentals, technology and applications. Wiley, New York, vol 3, part 2, pp 190–201.
Ratnasamy Ch, Wagner JP (2009) Water gas shift catalysis. Catal Rev 51:325–440
Liu B, Zhao L, Wu Zh, Zhang J, Zong Q, Almegren H, Wei F, Zhang X, Zha Zh, Gao J, Xiao T (2019) Recent advances in industrial sulfur tolerant water gas shift catalysts for syngas hydrogen enrichment: application of lean (low) steam/gas ratio. Catalysts 9(772):1–18
Nikolova D, Edreva-Kardjieva R, Gouliev G, Grozeva T, Tzvetkov P (2006) The state of (K)(Ni)Mo/g-Al2O3 catalysts after water–gas shift reaction in the presence of sulfur in the feed: XPS and EPR study. Appl Catal A 297:135–144
Nikolova D, Edreva-Kardjieva R, Grozeva T (2011) Water–gas shift activity of K-promoted (Ni)Mo/γ-Al2O3 systems in sulfur-containing feed. React Kinet Mech Cat 103:71–86
Nikolova D, Edreva-Kardjieva R, Serwicka E, Dula R, Grozeva T (2014) State of the components of (K)(Ni)W/γ-Al2O3 catalysts as oxide precursors and after water-gas shift reaction in the presence of sulfur. Appl Catal A 480:108–119
Nikolova D, Vakros J, Grozeva T, Lycourghiotis A, Tyuliev G, Edreva-Kardjieva R, Kordulis Ch (2015) Effect of tungsten deposition method on K-modified NiW/γ-Al2O3 as sulfur-tolerant water–gas shift reaction catalyst. Appl Catal A 506:14–24
Nikolova D, Edreva-Kardjieva R, Kolev H, Gabrovska M (2019) Promoted Re/Al2O3 systems as sour water-gas shift catalysts. Catal Today. https://doi.org/10.1016/j.cattod.2019.05.038
Andreev AA, Kafedjiysky VJ, Edreva-Kardjieva RM (1999) Active forms for water-gas shift reaction on NiMo-sulfde catalyst. Appl Catal 179:223–228
Nikolova D, Edreva-Kardjieva R, Giurginca M, Meghea A, Vakros J, Voyiatzis GA, Kordulis Ch (2007) The effect of potassium addition on the state of the components in the oxide precursor of the (Ni)(Mo)/γ-Al2O3 water-gas shift catalysts: FT-IR, diffuse reflectance and raman spectroscopic studies. Vib Spectrosc 44:343–350
Reinhoudt HR, Crezee E, van Langeveld AD, Kooyman PJ, van Veen JAR, Moulijn JA (2000) Characterization of the active phase in NiW/γ-Al2O3 catalysts in various stages of sulfidation with FTIR(NO) and XPS. J Catal 196:315–329
Scheffer B, Heijeinga JJ, Moulijn JA (1987) An electron spectroscopy and X-ray diffraction study of nickel oxide/alumina and nickel-oxide-tungsten trioxide/alumina catalysts. J Phys Chem 91:4752–4759
Schmitz-Du Mont O, Lule A, Reinen D (1965) Farbe und Konstitution bei anorganischen Feststoffen. VIII [1] A. Spektralphotometrische Ermittlung der Kationenverteilung im nickelhaltigen Magnesiumaluminiumspinell. Ber Bunsenges Phys Chem 69:76–81
LoJacono M, Schiavello M, Cimino A (1971) Structural, magnetic, and optical properties of nickel oxide supported on eta- and gamma-aluminas. J Phys Chem 75(8):1044–1050
Ashly JA, Mitchell CH (1968) Cobalt–molybdenum–alumina hydrodesulfurisation catalysts. Part I. A spectroscopic and magnetic study of the fresh catalyst and model compounds. J Chem Soc A 11:2821–2827
Ojeda J, Escalona N, Palacios JM, Yates M, Fierro JLG, López Agudo A, Gil Llambías FJ (2008) Promoter effect of Co on the catalytic activity of Re/γ-Al2O3 catalysts for the HDS and HDN of gas oil. Appl Catal A: General 350:6–15
McClure DS (1962) Optical spectra of transition-metal ions in corundum. J Chem Phys 36:2757–2762
van Deelen TW, Mejía CH, de Jong KP (2019) Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat Catal 2:955–970
Vakros J, Bourikas K, Kordulis Ch, Lycourghiotis A (2003) Influence of the impregnation pH on the surface characteristics and the catalytic activity of the Mo/γ-Al2O3 and CoMo/γ-Al2O3 hydrodesulfurization catalysts prepared by equilibrium deposition filtration (EDF). J Phys Chem B 107:1804–1813
Heracleous E, Vakros J, Lemonidoua AA, Kordulis Ch (2004) Role of preparation parameters on the structure–selectivity properties of MoO3/Al2O3 catalysts for the oxidative dehydrogenation of ethane. Catal Today 91–92:289–292
Hou P, Meerer D, Wise H (1983) Kinetic studies with a sulfur-tolerant water gas shift catalyst. J Catal 80:280–285
Lund CRF (1996) Effect of Adding Co to MoS2/Al2O3 upon the Kinetics of the Water−Gas Shift. Ind Eng Chem Res 35:3067–3073
Author information
Authors and Affiliations
Contributions
DN: conceptualization, synthesis and investigations, visualization, writing—original draft and editing. RE-K: DRS investigation, methodology, review and discussion. MG: XRD investigation, review and discussion.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nikolova, D., Edreva-Kardjieva, R. & Gabrovska, M. Catalytic performance stability of Mo, W and Re-based sour water–gas shift catalysts. Reac Kinet Mech Cat 130, 797–812 (2020). https://doi.org/10.1007/s11144-020-01801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01801-z