Abstract
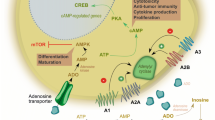
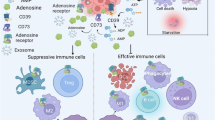
Cancer has the ability to escape the immune system using different molecular actors. Adenosine is known to be involved in mechanisms which control inflammatory reactions and prevent excessive immune response. This purine nucleoside can be translocated from the cell or produced in the extracellular space by 5′-ectonucleotidases. Once bound to its receptors on the surface of immune effector cells, adenosine activates various molecular pathways, which lead to functional inhibition of the cell or its death. Some tumors are infiltrated by the different cells of immune system but are able to use adenosine as an immunosuppressive molecule and thus inhibit immune anticancer response. This mechanism is well described on adaptive cells, but much less on innate cells. This review outlines major effects of adenosine on innate immune cells, its consequences on cancer progression, and possible ways to block the adenosine-dependent immunosuppressive effect.

Similar content being viewed by others
References
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867. https://doi.org/10.1038/nature01322
Barnes TA, Amir E (2017) HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer 117:451–460. https://doi.org/10.1038/bjc.2017.220
Kumar V (2013) Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signal 9:145–165. https://doi.org/10.1007/s11302-012-9349-9
Karmouty-Quintana H, Xia Y, Blackburn MR (2013) Adenosine signaling during acute and chronic disease states. J Mol Med 91:173–181. https://doi.org/10.1007/s00109-013-0997-1
Bester AC, Roniger M, Oren YS, Im MM, Sarni D, Chaoat M, Bensimon A, Zamir G, Shewach DS, Kerem B (2011) Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 145:435–446. https://doi.org/10.1016/j.cell.2011.03.044
Boison D, Yegutkin GG (2019) Adenosine metabolism: emerging concepts for cancer therapy. Cancer Cell 36:582–596. https://doi.org/10.1016/j.ccell.2019.10.007
Zylka MJ, Sowa NA, Taylor-Blake B, Twomey MA, Herrala A, Voikar V, Vihko P (2008) Prostatic acid phosphatase is an ectonucleotidase and suppresses pain by generating adenosine. Neuron 60:111–122. https://doi.org/10.1016/j.neuron.2008.08.024
Borea PA, Gessi S, Merighi S, Vincenzi F, Varani K (2018) Pharmacology of adenosine receptors: the state of the art. Physiol Rev 98:1591–1625. https://doi.org/10.1152/physrev.00049.2017
Horenstein AL, Bracci C, Morandi F, Malavasi F (2019) CD38 in adenosinergic pathways and metabolic re-programming in human multiple myeloma cells: in-tandem insights from basic science to therapy. Front Immunol 10:760. https://doi.org/10.3389/fimmu.2019.00760
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899. https://doi.org/10.1016/j.cell.2010.01.025
Haskó G, Pacher P (2012) Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol 32:865–869. https://doi.org/10.1161/ATVBAHA.111.226852
Najar HM, Ruhl S, Bru-Capdeville AC, Peters JH (1990) Adenosine and its derivatives control human monocyte differentiation into highly accessory cells versus macrophages. J Leukoc Biol 47:429–439. https://doi.org/10.1002/jlb.47.5.429
Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M (2011) A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. JI 186:2444–2453. https://doi.org/10.4049/jimmunol.1001567
Haskó G, Szabó C, Németh ZH et al (1996) Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 157:4634–4640
Xaus J, Mirabet M, Lloberas J et al (1999) IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: a mechanism of macrophage deactivation. J Immunol 162:3607–3614
Ferrante CJ, Pinhal-Enfield G, Elson G, Cronstein BN, Hasko G, Outram S, Leibovich SJ (2013) The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Rα) signaling. Inflammation 36:921–931. https://doi.org/10.1007/s10753-013-9621-3
Pinhal-Enfield G, Ramanathan M, Hasko G, Vogel SN, Salzman AL, Boons GJ, Leibovich SJ (2003) An angiogenic switch in macrophages involving synergy between toll-like receptors 2, 4, 7, and 9 and adenosine A2A receptors. Am J Pathol 163:711–721. https://doi.org/10.1016/S0002-9440(10)63698-X
Velot E, Haas B, Léonard F, Ernens I, Rolland-Turner M, Schwartz C, Longrois D, Devaux Y, Wagner DR (2008) Activation of the adenosine-A3 receptor stimulates matrix metalloproteinase-9 secretion by macrophages. Cardiovasc Res 80:246–254. https://doi.org/10.1093/cvr/cvn201
Csóka B, Selmeczy Z, Koscsó B, Németh ZH, Pacher P, Murray PJ, Kepka-Lenhart D, Morris SM Jr, Gause WC, Leibovich SJ, Haskó G (2012) Adenosine promotes alternative macrophage activation via A 2A and A 2B receptors. FASEB J 26:376–386. https://doi.org/10.1096/fj.11-190934
Young A, Ngiow SF, Gao Y, Patch AM, Barkauskas DS, Messaoudene M, Lin G, Coudert JD, Stannard KA, Zitvogel L, Degli-Esposti MA, Vivier E, Waddell N, Linden J, Huntington ND, Souza-Fonseca-Guimaraes F, Smyth MJ (2018) A2AR adenosine signaling suppresses natural killer cell maturation in the tumor microenvironment. Cancer Res 78:1003–1016. https://doi.org/10.1158/0008-5472.CAN-17-2826
Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E (2006) Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res 66:7758–7765. https://doi.org/10.1158/0008-5472.CAN-06-0478
Miller JS, Cervenka T, Lund J et al (1999) Purine metabolites suppress proliferation of human NK cells through a lineage-specific purine receptor. J Immunol 162:7376–7382
Neo SY, Yang Y, Julien R et al (2019) CD73 immune checkpoint defines regulatory NK-cells within the tumor microenvironment. J Clin Investig 130:1185–1198. https://doi.org/10.1172/JCI128895
Wu S, Awaji S (2019) Tumor-associated neutrophils in cancer: going pro. Cancers 11:564. https://doi.org/10.3390/cancers11040564
Felsch A, Stöcker K, Borchard U (1995) Phorbol ester-stimulated adherence of neutrophils to endothelial cells is reduced by adenosine A2 receptor agonists. J Immunol 155:333–338
McColl SR, St-Onge M, Dussault A-A et al (2006) Immunomodulatory impact of the A 2A adenosine receptor on the profile of chemokines produced by neutrophils. FASEB J 20:187–189. https://doi.org/10.1096/fj.05-4804fje
Bouma MG, Jeunhomme TM, Boyle DL et al (1997) Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J Immunol 158:5400–5408
Gessi S, Varani K, Merighi S, Cattabriga E, Iannotta V, Leung E, Baraldi PG, Borea PA (2002) A(3) adenosine receptors in human neutrophils and promyelocytic HL60 cells: a pharmacological and biochemical study. Mol Pharmacol 61:415–424. https://doi.org/10.1124/mol.61.2.415
Gorzalczany Y, Akiva E, Klein O, Merimsky O, Sagi-Eisenberg R (2017) Mast cells are directly activated by contact with cancer cells by a mechanism involving autocrine formation of adenosine and autocrine/paracrine signaling of the adenosine A3 receptor. Cancer Lett 397:23–32. https://doi.org/10.1016/j.canlet.2017.03.026
Gorzalczany Y, Merimsky O, Sagi-Eisenberg R (2019) Mast cells are directly activated by cancer cell–derived extracellular vesicles by a CD73- and adenosine-dependent mechanism. Transl Oncol 12:1549–1556. https://doi.org/10.1016/j.tranon.2019.08.005
Coillard A, Segura E (2019) In vivo differentiation of human monocytes. Front Immunol 10:1907. https://doi.org/10.3389/fimmu.2019.01907
Panther E, Idzko M, Herouy Y et al (2001) Expression and function of adenosine receptors in human dendritic cells. FASEB J 15:1963–1970. https://doi.org/10.1096/fj.01-0169com
Panther E, Corinti S, Idzko M, Herouy Y, Napp M, la Sala A, Girolomoni G, Norgauer J (2003) Adenosine affects expression of membrane molecules, cytokine and chemokine release, and the T-cell stimulatory capacity of human dendritic cells. Blood 101:3985–3990. https://doi.org/10.1182/blood-2002-07-2113
Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, Blackburn MR, Biaggioni I, Carbone DP, Feoktistov I, Dikov MM (2008) Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 112:1822–1831. https://doi.org/10.1182/blood-2008-02-136325
Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MKK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M (2006) A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci 103:13132–13137. https://doi.org/10.1073/pnas.0605251103
Fong L, Hotson A, Powderly JD, Sznol M, Heist RS, Choueiri TK, George S, Hughes BGM, Hellmann MD, Shepard DR, Rini BI, Kummar S, Weise AM, Riese MJ, Markman B, Emens LA, Mahadevan D, Luke JJ, Laport G, Brody JD, Hernandez-Aya L, Bonomi P, Goldman JW, Berim L, Renouf DJ, Goodwin RA, Munneke B, Ho PY, Hsieh J, McCaffery I, Kwei L, Willingham SB, Miller RA (2020) Adenosine 2A receptor blockade as an immunotherapy for treatment-refractory renal cell cancer. Cancer Discov 10:40–53. https://doi.org/10.1158/2159-8290.CD-19-0980
Antonioli L, Yegutkin GG, Pacher P, Blandizzi C, Haskó G (2016) Anti-CD73 in cancer immunotherapy: awakening new opportunities. Trends in Cancer 2:95–109. https://doi.org/10.1016/j.trecan.2016.01.003
Perrot I, Michaud H-A, Giraudon-Paoli M et al (2019) Blocking antibodies targeting the CD39/CD73 immunosuppressive pathway unleash immune responses in combination cancer therapies. Cell Rep 27:2411–2425.e9. https://doi.org/10.1016/j.celrep.2019.04.091
Woo S-R, Corrales L, Gajewski TF (2015) Innate immune recognition of cancer. Annu Rev Immunol 33:445–474. https://doi.org/10.1146/annurev-immunol-032414-112043
Vidyarthi A, Khan N, Agnihotri T, Negi S, Das DK, Aqdas M, Chatterjee D, Colegio OR, Tewari MK, Agrewala JN (2018) TLR-3 stimulation skews M2 macrophages to M1 through IFN-αβ signaling and restricts tumor progression. Front Immunol 9:1650. https://doi.org/10.3389/fimmu.2018.01650
Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, Holland EC, Stephan MT (2019) Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun 10:3974. https://doi.org/10.1038/s41467-019-11911-5
Zaghi E, Calvi M, Marcenaro E, Mavilio D, di Vito C (2019) Targeting NKG2A to elucidate natural killer cell ontogenesis and to develop novel immune-therapeutic strategies in cancer therapy. J Leukoc Biol 105:1243–1251. https://doi.org/10.1002/JLB.MR0718-300R
Saxena M, Bhardwaj N (2018) Re-emergence of dendritic cell vaccines for cancer treatment. Trends Cancer 4:119–137. https://doi.org/10.1016/j.trecan.2017.12.007
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16:183–194. https://doi.org/10.1016/j.ccr.2009.06.017
García-Rocha R, Monroy-García A, Hernández-Montes J, Weiss-Steider B, Gutiérrez-Serrano V, del Carmen Fuentes-Castañeda M, Ávila-Ibarra LR, Don-López CA, Torres-Pineda DB, de Lourdes Mora-García M (2019) Cervical cancer cells produce TGF-β1 through the CD73-adenosine pathway and maintain CD73 expression through the autocrine activity of TGF-β1. Cytokine 118:71–79. https://doi.org/10.1016/j.cyto.2018.09.018
Funding
LPJ receives funding from Olav Raagholt og Gerd Meidel Raagholts stiftelse for forskning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Regina Strakhova declares that she has no conflict of interest.
Octavia Cadassou declares that she has no conflict of interest.
Emeline Cros-Perrial declares that she has no conflict of interest.
Lars Petter Jordheim declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Strakhova, R., Cadassou, O., Cros-Perrial, E. et al. Regulation of tumor infiltrated innate immune cells by adenosine. Purinergic Signalling 16, 289–295 (2020). https://doi.org/10.1007/s11302-020-09701-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-020-09701-6