Abstract
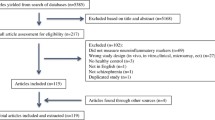
Although the neurobiological basis for autism spectrum disorder (ASD) has not yet been fully clarified, converging lines of evidence implicated a role of neuroinflammation in the etiological pathway of this disorder. The present article provided a systematic review of publications regarding the involvement of different components of neuroinflammation in postmortem brain samples of subjects diagnosed with ASD. A systematic search of PubMed, Embase, and Web of Science was conducted, which was supplemented by manual searching of reference lists of included articles. The screening for study and extraction of data were conducted by two independent authors after reviewing the abstract and full text. Of 356 articles identified in the literature search, 27 articles comprising 685 subjects (ASD = 313, controls = 351, schizophrenia = 10, epilepsy = 11) covering 19 brain regions met the eligibility criteria for this review. The search yielded 11 studies that estimated astrocyte-related changes, 8 studies that reported microglia-related changes, 2 studies that evaluated oligodendrocyte-related changes, 3 studies that examined changes in glial cells without differentiating cell types, 6 studies that evaluated the levels of cytokines and chemokines, and 7 studies that measured other inflammatory parameters in postmortem brain samples of subjects with ASD compared with controls. Although a few studies noted a lack of changes in neuroinflammatory markers in postmortem brain samples of ASD subjects, the majority of studies supported the presence of neuroinflammation in the neurobiological pattern of ASD as shown by activation of astrocytes and microglia together with abnormal levels of cytokines and chemokines.

Similar content being viewed by others
References
Almehmadi KA, Tsilioni I, Theoharides TC. (2020)Increased Expression of miR-155p5 in Amygdala of Children With Autism Spectrum Disorder. Autism Res. 13(1):18–23
Arikawa M, Kakinuma Y, Noguchi T et al (2016) Donepezil, an acetylcholinesterase inhibitor, attenuates LPS-induced inflammatory response in murine macrophage cell line RAW 264.7 through inhibition of nuclear factor kappa B translocation. Eur J Pharmacol 789:17–26
Ashwood P, Enstrom A, Krakowiak P et al (2008) Decreased transforming growth factor beta1 in autism: a potential link between immune dysregulation and impairment in clinical behavioral outcomes. J Neuroimmunol 204:149–153
Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, van de Water J (2011) Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun 25:40–45
Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W et al (2018) Prevalence of autism spectrum disorder among children aged 8 years-autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 67:1–23
Broek JAC, Guest PC, Rahmoune H, Bahn S (2014) Proteomic analysis of post mortem brain tissue from autism patients: evidence for opposite changes in prefrontal cortex and cerebellum in synaptic connectivity-related proteins. Mol Autism. 5(1):41
Cao F, Yin A, Wen G, Sheikh AM, Tauqeer Z, Malik M, Nagori A, Schirripa M et al (2012) Alteration of astrocytes and Wnt/β-catenin signaling in the frontal cortex of autistic subjects. J Neuroinflammation 9(1):223
Chana G, Laskaris L, Pantelis C, Gillett P, Testa R, Zantomio D, Burrows EL, Hannan AJ et al (2015) Decreased expression of mGluR5 within the dorsolateral prefrontal cortex in autism and increased microglial number in mGluR5 knockout mice: pathophysiological and neurobehavioral implications. Brain Behav Immun 49:197–205
Chez MG, Dowling T, Patel PB, Khanna P, Kominsky M (2007) Elevation of tumor necrosis factor-alpha in cerebrospinal fluid of autistic children. Pediatr Neurol 36:361–365
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 504:394–400
Clarke LE, Barres BA (2013) Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci 14:311–321
Courchesne E, Pierce K (2005) Brain overgrowth in autism during a critical time in development: implications for frontal pyramidal neuron and interneuron development and connectivity. Int J Dev Neurosci 23:153–170
Courchesne E, Carper R, Akshoomoff N (2003) Evidence of brain overgrowth in the first year of life in autism. JAMA. 290:337–344
Crawford JD, Chandley MJ, Szebeni K, Szebeni A, Waters B, Ordway GA (2015) Elevated GFAP protein in anterior cingulate cortical white matter in males with autism spectrum disorder. Autism Res 8(6):649–657
DiStasio MM, Nagakura I, Nadler MJ et al (2019) T lymphocytes and cytotoxic astrocyte blebs correlate across autism brains. Ann Neurol 86(6):885–898
Dong H, Zhang W, Zeng X, Hu G, Zhang H, He S, Zhang S (2014) Histamine induces up-regulated expression of histamine receptors and increases release of inflammatory mediators from microglial. Mol Neurobiol 49:1487–1500
Edmonson C, Ziats MN, Rennert OM (2014) Altered glial marker expression in autistic post-mortem prefrontal cortex and cerebellum. Mol Autism 5(1):3
Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcín C, Montiel-Nava C, Patel V et al (2012) Global prevalence of autism and other pervasive developmental disorders. Autism Res 5(3):160–179
Enstrom AM, Lit L, Onore CE et al (2005) Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry 17:485–495
Enstrom AM, Onore CE, Van de Water JA et al (2010) Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun 24:64–71
Fatemi SH, Folsom TD, Reutiman TJ, Lee S (2008) Expression of astrocytic markers aquaporin 4 and connexin 43 is altered in brains of subjects with autism. Synapse. 62(7):501–507
Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N et al (2016) Blood–brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 7(1):49
Garbett K, Ebert PJ, Mitchell A, Lintas C, Manzi B, Mirnics K, Persico AM (2008) Immune transcriptome alterations in the temporal cortex of subjects with autism. Neurobiol Dis 30(3):303–311
Giuliani C, Napolitano G, Bucci I, Montani V, Monaco F (2001) NF-kB transcription factor: role in the pathogenesis of inflammatory, autoimmune, and neoplastic diseases and therapy implications. La Clinica Terapeutica 152(4):249–253
Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH (2010) Mechanisms underlying inflammation in neurodegeneration. Cell. 140:918–934
Handen BL, Johnson CR, McAuliffe-Bellin S, Murray PJ, Hardan AY (2011) Safety and efficacy of donepezil in children and adolescents with autism: neuropsychological measures. J Child Adolesc Psychopharmacol 21(1):43–50
Hansen S, Schendel D, Parner E (2015) Explaining the increase in the prevalence of autism spectrum disorders: the proportion attributable to changes in reporting practice. JAMA Pediatr 169(1):56–62
Hashim H, Abdelrahman H, Mohammed D, Karam R (2013) Association between plasma levels of transforming growth factor-β1, IL-23 and IL-17 and the severity of autism in Egyptian children. Res Autism Spectrum Disord 7:199–204
Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ, Elison JT, Swanson MR et al (2017) Early brain development in infants at high risk for autism spectrum disorder. Nature. 542(7641):348–351
Karvat G, Kimchi T (2014) Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology. 39(4):831–840
Kim JW, Seung H, Kwon KJ, Ko MJ, Lee EJ, Oh HA, Choi CS, Kim KC et al (2014) Subchronic treatment of donepezil rescues impaired social, hyperactive, and stereotypic behavior in valproic acid-induced animal model of autism. PLoS One 9(8):e104927
Krakowiak P, Goines PE, Tancredi DJ, Ashwood P, Hansen RL, Hertz-Picciotto I, van de Water J (2017) Neonatal cytokine profiles associated with autism spectrum disorder. Biol Psychiatry 81:442–451
Krueger DD, Bear MF (2011) Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu Rev Med 62:411–429
Lange N, Travers BG, Bigler ED, Prigge MBD, Froehlich AL, Nielsen JA, Cariello AN, Zielinski BA et al (2015) Longitudinal volumetric brain changes in autism spectrum disorder ages 6-35 years. Autism Res 8(1):82–93
Laurence JA, Fatemi SH (2005) Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 4:206–210
Lee AS, Azmitia EC, Whitaker-Azmitia PM (2017a) Developmental microglial priming in postmortem autism spectrum disorder temporal cortex. Brain Behav Immun 62:193–202
Lee TT, Skafidas E, Dottori M, Zantomio D, Pantelis C, Everall I, Chana G (2017b) No preliminary evidence of differences in astrocyte density within the white matter of the dorsolateral prefrontal cortex in autism. Mol Autism. 8(1):64
Li X, Chauhan A, Sheikh AM, Patil S, Chauhan V, Li XM, Ji L, Brown T et al (2009) Elevated immune response in the brain of autistic patients. J Neuroimmunol 207:111–116
Masi A, Glozier N, Dale R, Guastella AJ (2017) The immune system, cytokines, and biomarkers in autism spectrum disorder. Neurosci Bull 33:194–204
Menassa DA, Sloan C, Chance SA (2017) Primary olfactory cortex in autism and epilepsy: increased glial cells in autism. Brain Pathol 27(4):437–448
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Morgan JT, Chana G, Pardo CA, Achim C, Semendeferi K, Buckwalter J, Courchesne E, Everall IP (2010) Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol Psychiatry 68(4):368–376
Morgan JT, Chana G, Abramson I et al (2012) Abnormal microglial–neuronal spatial organization in the dorsolateral prefrontal cortex in autism. Brain Res 1456(none):72–81
Morgan JT, Barger N, Amaral DG, Schumann CM (2014) Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder. PLoS One 9(10):e110356
Najjar S, Pearlman DM, Alper K et al (2013) Neuroinflammation and psychiatric illness. J Neuroinflammation 10:43
Nakanishi H, Hayashi Y, Wu Z (2011) The role of microglial mtDNA damage in age-dependent prolonged LPS-induced sickness behavior. Neuron Glia Biol 28:1–7
Napolioni V, Ober-Reynolds B, Szelinger S et al (2013) Plasma cytokine profiling in sibling pairs discordant for autism spectrum disorder. J Neuroinflammation 10:38
Norden DM, Godbout JP (2013) Microglial of the aged brain: primed to be activated and resistant to regulation. Neuropathol Appl Neurobiol 39(1):19–34
Okada K, Hashimoto K, Iwata Y, Nakamura K, Tsujii M, Tsuchiya KJ, Sekine Y, Suda S et al (2007) Decreased serum levels of transforming growth factor-beta1 in patients with autism. Prog Neuro-Psychopharmacol Biol Psychiatry 31:187–190
Owen DR, Narayan N, Wells L, Healy L, Smyth E, Rabiner EA, Galloway D, Williams JB et al (2017) Pro-inflammatory activation of primary microglial and macrophages increases 18 kDa translocator protein expression in ro-dents but not humans. J Cereb Blood Flow Metab 37(8):2679–2690
Pardo CA, Vargas DL, Zimmerman AW (2005) Immunity, neuroglia and neuroinflammation in autism. Int Rev Psychiatry 17:485–495
Patel N, Crider A, Pandya CD, Ahmed AO, Pillai A (2016) Altered mRNA levels of glucocorticoid receptor, mineralocorticoid receptor, and co-chaperones (FKBP5 and PTGES3) in the middle frontal gyrus of autism spectrum disorder subjects. Mol Neurobiol 53(4):2090–2099
Purcell AE, Jeon OH, Zimmerman AW, Blue ME, Pevsner J (2001) Postmortem brain abnormalities of the glutamate neurotransmitter system in autism. Neurology. 57(9):1618–1628
Rodriguez JI, Kern JK (2012) Evidence of microglial activation in autism and its possible role in brain underconnectivity. Neuron Glia Biol 7(2–4):1–9
Rose D, Ashwood P (2014) Potential cytokine biomarkers in autism spectrum disorders. Biomark Med 8:1171–1181
Rose S, Melnyk S, Pavliv O, Bai S, Nick TG, Frye RE, James SJ (2012) Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Transl Psychiatry 2(7):e134–e134
Rutter M (2005) Incidence of autism spectrum disorders: changes over time and their meaning. Acta Paediatr 94:2–15
Sahu JK, Gulati S, Sapra S, Arya R, Chauhan S, Chowdhury MR, Gupta N, Kabra M et al (2013) Effectiveness and safety of donepezil in boys with fragile X syndrome: a double-blind, randomized, controlled pilot study. J Child Neurol 28(5):570–575
Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, Wynshaw-Boris A, Colamarino SA et al (2014) Patches of disorganization in the neocortex of children with autism. N Engl J Med 370(13):1209–1219
Streit WJ, Mrak RE, Griffin WS (2004) Microglial and neuroinflammation: a pathological perspective. J Neuroinflammation 1(1):14
Tetreault NA, Hakeem AY, Jiang S, Williams BA, Allman E, Wold BJ, Allman JM (2012) Microglial in the cerebral cortex in autism. J Autism Dev Disord 42(12):2569–2258
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57(1):67–81
Wegiel J, Kuchna I, Nowicki K, Imaki H, Wegiel J, Yong Ma S, Azmitia EC, Banerjee P et al (2013) Contribution of olivofloccular circuitry developmental defects to atypical gaze in autism. Brain Res 1512:106–122
Wei H, Zou H, Sheikh AM, Malik M, Dobkin C, Brown WT, Li X (2011) IL-6 is increased in the cerebellum of autistic brain and alters neural cell adhesion, migration and synaptic formation. J Neuroinflammation 8(1):52
Weintraub K (2011) The prevalence puzzle: autism counts. Nature. 479(7371):22–24
Wright C, Shin JH, Rajpurohit A, Deep-Soboslay A, Collado-Torres L, Brandon NJ, Hyde TM, Kleinman JE et al (2017) Altered expression of histamine signaling genes in autism spectrum disorder. Transl Psychiatry 7(5):e1126–e1126
Young A, Campbell E, Lynch S et al (2011) Aberrant NF-kappaB expression in autism spectrum condition: a mechanism for neuroinflammation. Front Psychiatry 2:27
Zeidán-Chuliá F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JCF (2014) The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev 38:160–172
Zimmerman AW, Jyonouchi H, Comi AM, Connors SL, Milstien S, Varsou A, Heyes MP (2005) Cerebrospinal fluid and serum markers of inflammation in autism. Pediatr Neurol 33:195–201
Funding
This research was funded by the National Natural Science Foundation of China (No. 81873806) and the National Natural Science Foundation of Hunan Province (No. 5142019JJ40437).
Author information
Authors and Affiliations
Contributions
All authors have read and agree to the published version of the manuscript. Xiaoli Liao conceived the manuscript idea, identified eligible studies, extracted data, drafted the manuscript, and critically reviewed the manuscript. Yiting Liu searched database, identified eligible studies, and critically reviewed the manuscript. Xi Fu searched database, extracted data, and critically reviewed the manuscript. Yamin Li conceived the manuscript idea, was responsible for funding acquisition, and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, X., Liu, Y., Fu, X. et al. Postmortem Studies of Neuroinflammation in Autism Spectrum Disorder: a Systematic Review. Mol Neurobiol 57, 3424–3438 (2020). https://doi.org/10.1007/s12035-020-01976-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01976-5