Abstract
Purpose
To explore the dynamic changes and correlation between CT imaging manifestations and cellular immunity of COVID-19.
Materials and methods
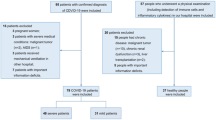
This retrospective review analyzed 23 patients with COVID-19, including 13 males and 10 females aged 27–70 years, with an average age of 48 years. Patients were divided into two groups: group A with 11 critical-severe patients, and group B with 12 common-mild patients. Clinical, laboratory, and radiological data were collected and analyzed.
Results
LYM, LYM (%), CD3+, CD4+, and CD8+ decreased, while NEU (%), CRP, and CT scores increased in all patients, WBC in group A increased. In group A, on day 10–12 after disease onset, CT scores and CRP reached the highest point, and day 13–15 LYM, LYM (%) reached the lowest but NEU (%) and WBC reached the highest, CD3+, CD4+ and CD8+ were at the lowest on day 10–15. In group B, on day 7–9, CT scores, NEU (%) and CRP reached the peak, but LYM, LYM (%), CD3+, CD4+ and CD8+ reached the lowest. In all patients, CT scores had a significantly negative correlation with CD3+, CD4+, CD8+, LYM (%), and LYM (p = 0.001, r = − 0.797; p = 0.008, r = − 0.698; p = 0.002, r = − 0.775; p < 0.001, r = − 0.785; p = 0.021, r = − 0.571, respectively), and a significantly positive correlation with WBC and NEU (%) (p < 0.001, r = 0.785; p = 0.003, r = 0.691, respectively).
Conclusion
Dynamic changes of CT manifestations and cellular immunity of patients with COVID-19 were regular and correlation was high between these two parameters.
Similar content being viewed by others
Introduction
Since late December 2019, coronavirus disease 2019 (COVID-19) broke out in Wuhan, China, and then swept across the country before spreading all over the world [1, 2]. COVID-19 is a newly discovered acute respiratory viral infectious disease with serious infectivity, which can be transmitted by droplets or contact [1, 2]. On January 30, 2020, the World Health Organization designated the epidemic of COVID-19 as a global health emergency.
At present, there is still a lack of effective treatment for the disease, and its outcome largely depends on the immune status of the patients themselves. In the clinic, the immune state of the human body, especially cellular immunity, is predicted by the results of peripheral blood test in the laboratory. The most common laboratory abnormalities include lymphopenia, decreased lymphocyte percentage, and abnormal T lymphocyte subsets, suggesting impaired cellular immune function which was very similar to SARS and MERS, indicating that viruses in the same virus family have a similar pathogenesis [3,4,5, 14, 15]. At the same time, pulmonary inflammation is the main manifestation of infected people, which is now called novel coronavirus pneumonia (NCP). Chest CT is used to evaluate the severity of lung involvement. Recent studies have found that NCP has characteristic CT manifestations in the lungs, and the images change dynamically with disease progression [6,7,8]. Therefore, it is important to determine whether the immune status of the human body or CT imaging manifestations, are closely related to the occurrence, development and outcome of COVID-19. To our knowledge, there are no current studies on the correlation between CT manifestations and cellular immunity in patients with COVID-19. The present study explored the dynamic changes of CT manifestations and cellular immunity in patients with COVID-19 to understand their underlying mechanisms and correlation, thus providing important reference information for early clinical diagnosis, course evaluation and treatment plan customization.
Materials and methods
Study design and participants
Chest CT images, laboratory findings, and clinical data were analyzed retrospectively for all patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes COVID-19, and confirmed by real-time polymerase chain reaction (RT-PCR) in our hospital from January 22 to February 21, 2020. Clinical results were monitored until the final follow-up date on February 21, 2020.There were 23 patients with COVID-19, including 13 males and 10 females aged 27–70 years, with an average age of 48 years (Table 1). Of these cases, 18 cases had a recent history of residing in or traveling to Wuhan and 5 cases had a history of close contact with confirmed cases. The main clinical manifestations in the 23 patients were fever and respiratory symptoms, expectoration, shortness of breath, and diarrhea (Table 1).
According to the standard of Notice on Diagnosis and Treatment of Novel Coronavirus Pneumonia (Trial Version 7) of the National Health Commission of the People's Republic of China, there are four clinical patient types [9]. Mild illness is defined by mild clinical symptoms, and no pneumonia found during imaging. Common illness is defined by clinical symptoms such as fever and respiratory tract, with pneumonia evident during imaging. Severe illness diagnostics are: (1) shortness of breath with respiratory rate ≥ 30 breaths/min; (2) SpO2 ≤ 93%; 3. PaO2/FiO2 ≤ 300 mmHg (1 mmHg = 0.133 kPa); and (4) lung imaging showing lesion progression ≥ 50% within 24–48 h. Critical illness is defined by: (1) occurence of respiratory failure and requirement of mechanical ventilation; (2) occurence of shock; (3) other organ failure and the need for ICU monitoring treatment. There were four critical type cases (17%), seven severe type cases (31%), nine common type cases (39%) and three mild type cases (13%) in the present study. Patients were divided into two groups: group A with 11 critical-severe patients with an average age of 50 years (7 males and 4 females), and group B with 12 common-mild patients with an average age of 46.17 years (6 males and 6 females). The mortality rate in two groups was 0%. The duration of hospitalization ranged from 18 to 30 days, with an average of 24 days in group A, which ranged from 12 to 28 days, with an average of 20 days in group B. The retrospective analysis was approved by the institutional review board and patient consent was waived.
Data collection
The immune status of the human body was predicted by results of laboratory peripheral blood tests. The laboratory parameters studied included white blood cell (WBC), lymphocyte (LYM), lymphocyte percentage, neutrophil (NEU) percentage, C-reactive protein (CRP) and CD3+, CD4+, and CD8+T cells counts. Detection of peripheral blood routine was performed using an automatic hematology analyzer (Mindray BC-5390) and detection of T lymphocyte subsets was performed using flow cytometry (BECKMAN/Navios10CLORS/3LASER). All laboratory results of each patient before and during admission were recorded and analyzed.
All patients underwent chest CT scan without intravenous contrast in the prescribed examination room before and during admission. Chest data were collected with ScintCare CT 16 (MingFeng, China) and DuoEmotion CT (Siemens, Germany), with a scan range from the tip of the lung to the bottom, and scan thickness of 2.5–5 mm, to reconstruct axial images with slice thickness of 1–1.25 mm. The images were read separately by two experienced radiologists in the cardiothoracic group. In case of disagreement, the two were agreed through consultation and reviewed by the third chief radiologist. CT findings were recorded, including lesion range, location, shape, density and extrapulmonary manifestations. The extent of lesion involvement in CT images was scored by semi-quantitative scoring system [6, 7]. The extent of lesion involvement was called a “CT score”, which was the sum of the range of five lobar lesions, ranging from 0 to 25 points. Each of the five lung lobes was visually scored from 0 to 5 as follows: 0, no involvement; 1, < 5% involvement; 2, 5–25% involvement; 3, 26–49% involvement; 4, 50–75% involvement; and 5, > 75% involvement.
Every patient underwent blood and CT examinations on an irregular schedule from onset, resulting in inconsistent timings, frequency and intervals. A time point of 3 days was used to calculate the average value of laboratory indicators and CT scores in each time period within 28 days.
Statistical analysis
All data were analyzed using SPSS25.0 statistical software. The counting data were expressed using frequency and rate, the measurement data were expressed using x ± s in accordance with a normal distribution, and the non-normal distribution was expressed using the median (quartile interval) [M(Q)]. The clinical features and CT scores of two groups were compared using χ2 or Fisher’s exact probability method. All laboratory parameters were compared using an independent sample t test. Spearman’s correlation analysis was used to calculate the correlation between CT scores and each laboratory index, and p < 0.05 was considered statistically significant.
Results
Dynamic changes of laboratory parameters
The laboratory indicators exhibited a changing trend with disease progression (Fig. 1). In the early stage of the disease, LYM, LYM (%), CD3+, CD4+, and CD8+ decreased, NEU (%) and CRP increased in both groups, while WBC increased in group A, and remained normal or decreased in group B. On day 10–12 after disease onset in group A, CRP reached its highest point, while LYM and LYM (%) reached their lowest points, NEU (%) and WBC reached their highest points on day 13–15. CD3+, CD4+ and CD8+ were at a low level in the early stage of the disease, and reached their lowest levels on day 10–15 after symptom onset. On day 7–9 after disease onset in group B, CRP and NEU (%) reached their highest points, while LYM, LYM (%), CD3+, CD4+, and CD8+ reached their lowest. All patients’ indicators tended to improve gradually in the later stage of the disease. There were significant differences in NEU (%), LYM, and LYM (%) between the two groups on day 7–18 (all p < 0.05, Table 2). On days 7–12, 16–18, and 25–27, there were significant differences in WBC between the two groups (all p < 0.05, Table 2). There were significant differences in CD3+ and CD8+ between the two groups on day 10–18 (all p < 0.05, Table 3). CD4+ in the two groups had significant differences on days 10–12 and 16–18 (both p < 0.05, Table 3). The average frequency and interval of laboratory tests are presented in Table 4.
Dynamic changes of laboratory parameters and CT scores in two groups of COVID-19 patients. Blue solid line: group A of critical-severe patients, yellow solid line: group B of common-mild patients. The points are the average values of laboratory indicators and CT scores for the 3-day intervals in two groups (colour figure online)
Dynamic changes of CT images
A total of 75 chest CT examinations were performed in 23 patients. Abnormalities were found in 70 times (94%) in 20 patients (87%). The average frequency and interval of CT examinations are presented in Table 4. CT results showed multiple instances of ground glass opacity (GGO) and/or consolidation on either both sides or one side, often involving the middle and outer zones of the lungs (Figs. 2, 3). The scope and density of pulmonary lesions increased, consolidation increased, and CT scores increased accordingly with disease progression (Table 5). In the absorption phase, the lesion area and density decreased, CT scores decreased, and pulmonary interstitial fibrosis appeared, which was characterized by scattered or multiple stripes, reticular shadow, and thickened interlobular septum (Figs. 2, 3). The CT scores of the whole lung involvement ranged from 4 to 21, with an average of 11.3 in group A, which ranged from 0 to 11, with a mean score of 4.8 in group B. On day 4–21, the CT scores in group A consisting of critical-severe patients were significantly higher than those in group B which consisted of common-mild patients (Table 5, all p < 0.05). The CT scores reached their peak on day 10–12 after symptom onset, and decreased gradually starting on day 22–24 in group A (Fig. 1). In group B, the CT scores reached their peak on day 7–9 after initial symptom onset, and then decreased gradually (Fig. 1). In group A, eight patients (35%) had pleural effusion and three patients (13%) had pericardial effusion.
Correlation between CT scores and laboratory parameters in all COVID-19 patients. a–g CT scores had a highly negative correlation with CD3+, CD4+, CD8+, LYM (%), and LYM (p = 0.001, r = − 0.797; p = 0.008, r = − 0.698; p = 0.002, r = − 0.775; p < 0.001, r = − 0.785; p = 0.021, r = − 0.571, respectively), and a highly positive correlation with WBC and NEU (%) (p < 0.001, r = 0.785; p = 0.003, r = 0.691, respectively). The points are the average values of laboratory indicators and CT scores for the 3-day intervals in two groups
Correlation between CT scores and laboratory parameters in critical-severe patients. a–c CT scores had a highly negative correlation with LYM (%) and LYM (p = 0.025, r = − 0.733, p = 0.03, r = − 0.717, respectively), and a highly positive correlation with NEU (%) (p = 0.025, r = 0.733). The points are the average values of laboratory indicators and CT scores for the 3-day intervals in group A
Correlation between laboratory results and CT image scores
The CT scores had a significantly negative correlation with CD3+, CD4+, CD8+, LYM (%), and LYM (p = 0.001, r = − 0.797; p = 0.008, r = − 0.698; p = 0.002, r = − 0.775; p < 0.001, r = − 0.785; p = 0.021, r = − 0.571, respectively), and a significantly positive correlation with WBC and NEU (%) (p < 0.001, r = 0.785; p = 0.003, r = 0.691, respectively) in all patients (Fig. 4). In group A, the CT scores had a significantly negative correlation with LYM (%) and LYM (p = 0.025, r = − 0.733, p = 0.03, r = − 0.717, respectively), and a significantly positive correlation with NEU (%) (p = 0.025, r = 0.733, Fig. 5).
Axial and coronal chest CT images of a critical female COVID-19 patient, 66 years old. (A1–A2) On day 12 after disease onset, there was extensive ground glass opacity (GGO) in both lungs with local consolidation and little pleural effusion on both sides. Laboratory results of LYM, WBC, LYM (%), NEU (%), and CRP were 0.54 × 109/L, 11.12 × 109/L, 4.9%, 89.2%, and 11.46 mg/L, respectively. B1–B2 On day 21, most of the lesions were absorbed and interlobular septum was thickened. There was no blood test
Axial and coronal chest CT images of a common male COVID-19 patient, 45 years old. A1–A2 On day 9 after disease onset, multiple instances of patchy GGO were scattered in both lungs and the interlobular septum was thickened in the lower lobe of the left lung. There was no blood test. B1–B2 On day 21, most of GGO in both lungs were absorbed, multiple interstitial fibrosis were scattered and interlobular septum was thickened in the lower lobe of both lungs. Laboratory results of LYM, WBC, LYM (%), and NEU (%) were 1.6 × 109/L, 8.29 × 109/L, 19.3%, and 70.4%, respectively
Discussion
SARS-CoV-2 belongs to lineage β of the genus betacoronavirus of the coronavirus family. Its main clinical manifestations include fever, dry cough, and fatigue. In severe cases, it can rapidly develop into acute respiratory distress syndrome (ARDS), septic shock, or multiple organ failures (MODS) [1,2,3,4,5]. The early stage of a high inflammatory response in patients is also known as a cytokine storm. In this stage, the body secretes a variety of cytokines and chemokines to produce a strong immune response and overactivated immune cells, such as neutrophils and macrophages, gather in the lungs, causing apoptosis and destruction of lung epithelial cells, while clearing the virus and leading to lung injury [10,11,12,13,14,15].
In this study, semi-quantitative calculation of the lesion area in CT images of pneumonia was used to objectively evaluate the degree of lung injury caused by COVID-19. The degree of pneumonia involvement in severe patients was significantly higher than that in non-severe patients, suggesting that there were individual differences in the degree of lung injury caused by COVID-19. In severe patients, diffuse alveolar damage with cellular fibromyxoid exudates, pulmonary edema, and hyperimmunity were more serious [5, 10]. This also implies that imaging manifestations may correspond to the clinical grade. Therefore, CT reexamination during the course of the disease is very important. According to the CT manifestations, the degree of lung injury can be timely evaluated in different patients at different stages to quickly formulate a corresponding treatment plan to inhibit excessive inflammation to control the disease progression.
Cellular immunity is a very important part of the immune system, which is regulated and maintained by T lymphocyte subsets. Studies have shown that the early excessive inflammatory response characterized by a cytokine storm in patients with severe infection was a type of immune hyperactivity, followed by depletion of immune cell apoptosis and a decrease in cellular immune function, which is a state of immunosuppression [10,11,12,13,14,15]. In the present study, WBC, NEU (%), and CRP increased to a varying degrees, while LYM, LYM (%), CD3+, CD4+, and CD8+ counts decreased especially in critical-severe patients with COVID-19. These results indicate that the immune balance of the body was broken, the cellular immune function was impaired, the body could not effectively eliminate coronavirus in time, and the degree of immunosuppression was related to the severity of the disease [13,14,15]. In our study, CD3+, CD4+ and CD8+ reached the peak on day 10–12 after onset, and then decreased in group B. We analysed the data found that there were three mild patients who recovered well, leading to the peak of the data. It is suggested that WBC, NEU (%), CRP, LYM, LYM (%), CD3+, CD4+, and CD8+T cell counts may be predictors of disease severity.
The present study showed that lung and human cellular immune injuries were the most serious in critical-severe patients from the second week to the third week, while lung injury and immune function were the worst in common-mild patients during the early second week. The results showed that in this period, the immune hyperactivity characterized by a cytokine storm and the cellular immunosuppression characterized by "T lymphopenia" occurred at the same time, and that disease progression reached a peak [10,11,12,13,14,15]. Therefore, the second to third week in the course of the disease was the key time for clinical intervention. To restore the body to the stage of immune homeostasis, it is necessary not only to use drugs to inhibit excessive inflammation or blood purification to remove cytokines and chemokines, but also to use immune enhancers such as thymosin and gamma globulin to improve cellular immune function, prevent virus proliferation and spread, and prevent pneumonia aggravation [9,10,11,12,13,14,15].
This study had some limitations: (1) only 23 patients were included in the study and the small number of samples in the outlined time periods may lead to large errors. (2) Due to the hospital rules, patients with fever who were suspected and diagnosed with COVID-19 were examined in different examination rooms, with different CT scanning equipments, resulting in different image thicknesses, and partial CT images bias. However, these factors do not affect the evaluation of the severity of lesions and disease diagnosis, which is what truly reflected the actual medical response in the epidemic area. (3) The variability and reproducibility (interobserver and intraobserver) of CT scores could not be tested. 4. The hospital did not have the equipment for detecting cytokines such as interleukin and could not directly confirm the occurrence of a cytokine storm.
In summary, the dynamic changes of CT imaging manifestations and status of cellular immunity in patients with COVID-19 were regular and showed different temporal patterns according to the severity of the disease. Although the functions of chest CT examination and blood tests are different and can not be replaced by each other, there was a high correlation between CT manifestations and cellular immune status. Monitoring CT examinations results and laboratory tests may be helpful for timely evaluation of lung injury severity and the state of cellular immunosuppression and for individually adjusting the treatment plan.
Abbreviations
- CT:
-
Computed tomography
- COVID-19:
-
Coronavirus disease 2019
- WBC:
-
White blood cell
- LYM:
-
Lymphocyte
- LYM (%):
-
Lymphocyte percentage
- NEU (%):
-
Neutrophil percentage
- CRP:
-
C-reactive protein
- GGO:
-
Ground glass opacity
References
Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506.
Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020. https://doi.org/10.1001/jama.2020.15854.
Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020. https://doi.org/10.1007/s11427-020-1643-8.
Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected by SARS-CoV-2 in Wuhan. China Allergy. 2020. https://doi.org/10.1111/all.14238.
Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020. https://doi.org/10.1016/S2213-2600(20)30076-X.
Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020. https://doi.org/10.1148/radiol.2020200370 (Epub ahead of print).
Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020. https://doi.org/10.1148/radiol.2020200230.
Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan. China Eur Radiol. 2020. https://doi.org/10.1007/s00330-020-06731-x.
National Health Commission of the People's Republic of China. Notice on diagnosis and treatment of novel coronavirus pneumonia (Trial Version 7) [EB/OL] (2020-03-03) . https://med.sina.cn/article_detail_103_1_78534.html (in Chinese)
Chen J, Lau YF, Lamirande EW, et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4 T cells are important in control of SARS-CoV infection. J Virol. 2010;84(3):1289–301. https://doi.org/10.1128/JVI.01281-09.
Gupta KK, Khan MA, Singh SK. Constitutive inflammatory cytokine storm:a major threat to human health [J]. J Interferon Cytokine Res. 2020;40(1):19–23. https://doi.org/10.1089/jir.2019.0085.
Tisoncik JR, Korth MJ, Simmons CP, et al. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. https://doi.org/10.1128/MMBR.05015-11.
Hotchkiss RS, Monneret G, Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13(12):862–74. https://doi.org/10.1038/nri3552.
Lam CW, Chan MH, Wong CK. Severe acute respiratory syndrome: clinical and laboratory manifestations. Clin Biochem Rev. 2004;25(2):121–32.
Channappanavar R, Zhao J, Perlman S. T-cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59(1–3):118–28. https://doi.org/10.1007/s12026-014-8534-z.
Funding
This work was supported by Zhejiang University Emergency Scientific Research Project for COVID-19 (2020XGZX036), Zhejiang Medical and Health Science and Technology Program Project (2020KY980), Zhejiang Public Welfare Technology Application Social Development Project (LGF20H180008), Shaoxing Health and Family Planning Science and Technology Innovation Project (2017CX008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there is no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yang, M., Mao, H., Chen, L. et al. Study on the dynamic change law and correlation between CT imaging manifestations and cellular immunity of 2019 novel coronavirus disease. Jpn J Radiol 38, 942–952 (2020). https://doi.org/10.1007/s11604-020-00997-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11604-020-00997-3