Abstract
The production of hydrogen to be used as an alternative renewable energy has been widely explored. Among various methods for producing hydrogen from hydrocarbons, methane decomposition is suitable for generating hydrogen with zero greenhouse gas emissions. The use of high temperatures as a result of strong carbon and hydrogen (C–H) bonds may be reduced by utilizing a suitable catalyst with appropriate catalyst support. Catalysts based on transition metals are preferable in terms of their activeness, handling, and low cost in comparison with noble metals. Further development of catalysts in methane decomposition has been investigated. In this review, the recent progress on methane decomposition in terms of catalytic materials, preparation method, the physicochemical properties of the catalysts and their performance in methane decomposition were presented. The formation of carbon as part of the reaction was also discussed.
Graphic abstract

Similar content being viewed by others
Introduction
The increase in greenhouse gas (GHG) emissions has intensified global warming. The continuous usage of fossil fuels has caused severe environmental pollution thus leading to research and development of alternative resources to overcome this problem for a cleaner environment [1,2,3]. Studies have been conducted to develop clean, renewable, and sustainable sources of energy, along with devices. One of the most favorable methods for dealing with current environmental crises and energy issues is fuel cell technologies. Fuel cells are electrochemical devices that convert hydrogen into heat and electricity and they are being developed for various applications [4, 5]. Hydrogen energy is becoming one of the alternative sources that can reduce fossil fuel consumption so that environmental pollution can be controlled. Hydrogen requirement in fuel cell technology has led to hydrogen production in many industrial applications. With the unique thermal properties of this technology, hydrogen can be used as transportation fuel [5]. This fuel cell technology is more convenient and greener than existing combustion fuels, such as natural gas and gasoline.
Several methods, including photocatalysis [6], water splitting [7, 8], steam reforming process [9,10,11,12,13], and hydrocarbon decomposition [14,15,16,17,18,19], have been developed to produce hydrogen. The commonly used method is steam reforming owing to its simplicity and efficiency [20, 21]. However, catalyst deactivation due to coke formation is a major issue in steam reforming [22, 23]. As a possible alternative to steam reforming, methane decomposition is an effective process to produce hydrogen because of its simplicity and low cost given that it does not require a water–gas shift (WGS) reaction for further hydrogen purification [24].
Among hydrocarbons studied for decomposition, methane decomposition is the most examined method because it can directly convert methane into hydrogen and carbon without producing any byproduct. Mondal and Chandran [25] and Zhang et al. [26] compared the production of hydrogen from methane decomposition and methane steam reforming. Methane steam reforming is widely used to produce a high amount of hydrogen. However, the presence of COx as a byproduct affects hydrogen purity [27]. Methane decomposition is preferable because it produces COx-free hydrogen. The water–gas shift (WGS) reaction or other purification processes are unnecessary in methane decomposition because CO and CO2 are not produced as byproducts of the reaction, resulting in a clean process [28].
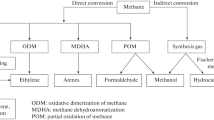
Methane decomposition is an endothermic process; hence, it requires high operational temperature at approximately 1200 °C without the help of the catalyst. A suitable and reliable catalyst can reduce the reaction temperature. Several important factors, such as catalyst support, metal loading, particle size, and reaction conditions, may affect the performance of a catalyst during methane decomposition [29,30,31,32,33,34,35,36,37,38,39,40,41]. Weger et al. [42] reported that methane decomposition produces economical hydrogen, which may alleviate climate change because approximately 27% of COx emission can be reduced by this direct decomposition. Figure 1 shows the pathway in conducting methane decomposition that involves selecting catalysts and reaction temperatures, designing reactors, and finally generating reaction products.

adopted from Keipi et al. [43]
Catalytic decomposition pathway in constructing the reaction parameter
This study provides an overview of hydrogen production via methane decomposition over heterogeneous catalysts. In terms of active components, nickel (Ni)-based catalyst is a common metal used in this process. The nature of catalyst support, preparation method, Ni metal loading on the support, and Ni modification by the addition of second and noble metals as promoters were also greatly examined in many studies. In addition to a Ni catalyst, other metals as a catalyst in methane decomposition have been deliberated.
Metal-based catalyst
Ni-based catalyst
Numerous studies have been performed on methane decomposition using a Ni-based catalyst because it is known as a favorable active metal in this catalytic reaction [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. Methane decomposition over Ni-based catalysts has been widely studied at a laboratory scale because of its low cost and effectiveness in the reaction, thus becoming a potential catalyst in practical and industrial applications. Lua and Wang [59] examined the unsupported Ni-based catalyst in methane decomposition and found that methane should be introduced to a reaction at a low temperature so that the sintering of the catalyst can be hindered.
Guil-Lopez et al. [60] compared the activities of metal- and carbon-based catalysts. Ni supported by MgAl mixed oxides obtained from hydrotalcite-like materials (Ni-ex LDH) is synthesized via co-precipitation, and a commercial carbon is used in this study. Catalytic testing has revealed that most active catalysts are Ni-ex LDH with higher initial activity than carbon-based ones. The activity of carbon catalysts is influenced by the number of defects present on the surface of the graphene layers. Numerous defects result in high catalytic activity in hydrogen production. Ni metal can also produce carbon nanofibers at an intermediate temperature [61]. Pinilla et al. [92] compared the activity of Ni/Al2O3 and carbon black in methane decomposition. The reaction was conducted in the fluidized bed reactor with the reaction temperature ranging from 600 to 950 °C and weight hourly space velocity (WHSV) used was in the range of 0.9–5.0 LN/(gcat h). The results showed that a high methane conversion (48%) is achieved by Ni/Al2O3, whereas a carbon black catalyst experiences a slight decrease in the initial methane conversion (34%) possibly because of the loss in surface functionalities of carbon black during the initial reaction stage. The equilibrium of methane conversion shifts toward the side of products as the reaction temperature increases because the overall process is highly endothermic. However, a Ni-based catalyst is rapidly deactivated because of the formation of encapsulated carbon over the catalyst surface at a high temperature even though it provides a high initial activity compared with Co- and Fe-based catalysts [93]. Hence, the selection of suitable catalyst support, the addition of a second metal, the amount of Ni loading, and the preparation of catalysts are keys to overcoming the problem of Ni-based catalysts. Table 1 summarizes mono- and bimetallic Ni-based catalysts that were used in the methane decomposition process supported by various materials.
Effect of support
One of the major contributors of catalytic activity in methane decomposition is the nature of catalyst supports. The main factor that contributes to catalytic activities is the proper interaction between an active metal and catalyst support [30]. The optimal support should exhibit favorable properties, such as chemical and mechanical resistance, and a high surface area to promote the enhanced dispersion of the active phase [31]. The acidity of the support improves the methane decomposition, and the catalysts become highly refractory and stable in sintering and coke formation because of the proper interaction between metals and supports [98]. Dou et al. [99] validated the presence of active sites on a catalyst’s surface significantly affects the reaction activity and reported that catalyst deactivation can be considered as a decrease in the number of active sites. Furthermore, Gupta [100] elaborated on the importance of the concentration of active site surfaces and the high surface area of catalyst supports. The decrease in methane conversion is due to the formation of encapsulating carbon, which hinders the access to active sites by covering their active surfaces. Bayat et al. have stated the encapsulating carbon occurs because of the imbalance between the amount of carbon produced at the metal/gas interface, the amount of carbon transferred through nickel particles, and its nucleation and deposition on the nickel/graphite side [101]. Hence, suitable catalyst support coupled with an appropriate preparation method can improve catalytic activity [102]. Catalyst support with a high surface area likely leads to a strong metal support interaction by providing an enhanced dispersion of metal active sites on the surface of a supporting catalyst, thus increasing the catalytic activity [40, 41].
Metal oxides, such as Al2O3, SiO2, MgO, CeO2, and TiO2, are frequently used as catalyst supports in methane decomposition [51, 73, 84, 94, 103,104,105,106]. Chesnokov and Chichkan [65] studied the performance of NiO supported by Al2O3 prepared by mechanochemical activation in methane decomposition. The results showed that the contact between metal particles has decreased because of the enhanced dispersion of aluminum oxide particles between metals, thereby hindering the metal sintering process and the particle detachment from the surface of the catalyst are depressed, in agreement with the study carried out by Frusteri et al. [67]. These results are observed at approximately 50–60% methane conversion at an optimum reaction temperature of 700–750 °C. The activity of a NiO–TiO2 hybrid, synthesized via sol–gel method, followed by calcination, is investigated by Shen and Lua [74]. The strong interaction between Ni and TiO2 led to the fine dispersion of Ni particles on the surface of TiO2. The presence of TiO2 support has modified the physicochemical properties of the catalysts, including surface area (172 m2/g), pore volume (0.55 cm3/g) and particle size (10–25 nm), resulted in a better catalytic activity in terms of catalyst stability and hydrogen formation rate, compared with unsupported Ni catalyst. The influence of composite catalyst supports, such as Al2O3/TiO2, on the catalytic activity of methane decomposition has also been studied [72]. The result has shown that the catalytic activity of Ni/Al2O3 increases because the formation of Ni aluminate (NiAl2O4) spinel structure is hindered by the introduction of TiO2. Thus, the catalyst reduction is assisted. The un-promoted catalyst shows high initial activity with 50% hydrogen yield, whereas Ni/Al2O3–TiO2 displays 34% of hydrogen yield during the initial reaction time. However, the activity of the latter catalyst increases to 56%, and no deactivation occurs, indicating that the incorporation of TiO2 improves catalytic stability. The temperature-programmed reduction (TPR) analyses support the hypothesis that an increase in TiO2 concentration enhances the dispersion of Ni particles. The enhanced dispersion of Ni nanoparticles on the support leads to the fast adsorption and enhances the solubility of methane molecules as more active sites of Ni become available, thereby speeding up methane decomposition [85].
Pudukudy et al. studied the catalytic activity of a Ni catalyst over various metal oxide supports, such as TiO2 [63], SiO2 [93], MgO [62], and MgAl2O4 [85]. Among all catalysts, 50 wt% Ni supported by SiO2 prepared through impregnation shows the highest activity with 74% of hydrogen yield. However, the stability of this catalyst is lower than that of other studied catalysts because of carbon agglomeration on the surface of the catalyst, thus blocking the availabilities of active sites on the surface of Ni/SiO2 catalysts. Ni/TiO2 shows optimal stability with 24% of hydrogen yield for 960 min on stream. The lifetime of the catalyst is long because of the fine dispersion of active Ni nanoparticles on TiO2 support. The same inference is applied to a Ni/MgO catalyst with enhanced catalyst stability within a 360 min reaction time, although the hydrogen yield (51%) is low. Ni/MgAl2O4 exhibits a low activity with 50% hydrogen yield because of the low surface area (22.6 m2/g) and the strong interaction between the aggregated NiO and the support, thereby causing less NiO species to be diminished as active sites. Nuernberg et al. [76] studied the effect of MgAl2O4 as a support for a Ni catalyst. The result showed that the activity of a Ni/MgAl2O4 catalyst is affected by the applied operating conditions. In this study, N2:CH4 ratio is varied, and the results have revealed that methane conversion increases as the N2:CH4 molar ratio increases. This finding is due to the inadequate availabilities of active sites on the catalyst surface to convert all reactive molecules. The boundary sites of the metal support are liable for the improvement of overall catalytic activity due to its ability to generate the reaction steps [107].
Awadallah et al. [71] investigated the effect of binary mixed oxides, such as SiO2–Al2O3, SiO2–La2O3, SiO2–MgO, and SiO2–CeO2, on the catalytic activity of a Ni catalyst in methane decomposition. The catalysts are prepared through wet impregnation with 50 wt% Ni loading on each supporting catalyst. The catalytic testing has shown the most active catalyst with the highest hydrogen yield is achieved by Ni/SiO2–Al2O3, whereas Ni/SiO2–CeO2 displays remarkable catalyst stability for 180 min on stream with moderate activity. This occurrence may be due to the presence of the facile reduction of NiO species in the catalyst and the fine dispersion of Ni particles on the supporting catalyst. Tapia-Parada et al. [75] investigated the effect of CeO addition on the Ni/SiO2 activity prepared via the surfactant-assistant sol–gel method. The P123 surfactant in this work is used to prevent the agglomeration of metallic Ni nanoparticles on support matrices. This surfactant can control the diameter and shape of metal particles. The methane conversion by the promoted catalyst is higher than that of Ni/SiO2 with a slow catalyst deactivation. CeO is allowed to stabilize active sites, thereby leading to the prevention of thermal loss on the surface area of the catalyst. Therefore, the stability of the catalyst for methane decomposition is improved. Pudukudy et al. [64] investigated the effect of metal oxides, such as lanthana, ceria, and zirconia, as catalyst support over a Ni catalyst. Among the studied catalysts, Ni supported by lanthana shows the highest catalytic stability with the highest amount of carbon formed during methane decomposition. This result is due to the finely crystallized Ni particles, which are dispersed on the support surface. The electronic promotion effect of La2O3 has increased the dissociation rate of C–H bond by electron charge transfer to nickel, which then led to the enhanced stability of the Ni-based catalyst in methane decomposition [97].
Although studies have been performed on metal oxide and carbonaceous materials as catalyst supports for methane decomposition, silica-based material possesses a high surface area, thereby making it favorable to be used as catalyst support. Many studies have focused on the use of silica as support materials. Mesoporous silica possesses a high surface area, high thermal stability, tunable pore size, and ordered porous network for the enhanced reactant diffusion [108]. The pore size of the catalyst support is also necessary to determine the performance of a catalyst [84]. Besides, the high surface area and pore size of mesoporous silica are well suited for the production of carbon nanotubes as reported by Palacio et al. [109], owing to the unique pore structure which may help in preventing the sintering of metal particles during the reaction by stabilizing the particles on the catalyst support. Tanggarnjanavalukul et al. [77] studied the effect of the pore structure of the mesoporous silica material over a Ni-based catalyst. They reported the properties of pore structures significantly influence the catalytic activity in methane decomposition. Bimodal porous silica support with straight (MCM-41) and sinusoidal pores (BPS-5) supported by a Ni catalyst are used. Although the surface area of Ni/BPS-5 is lower than that of Ni/MCM-41, methane conversion and catalyst stability are enhanced. The proper dispersion of Ni particles on BPS-5 has contributed to the enhanced activity in methane decomposition, and this observation is consistent with the results of Tsoncheva et al. [110]. Guevara et al. [78] investigated the performance of Ni/Ce-MCM-41 in methane decomposition. Catalytic testing has displayed high stability and methane conversion. The Ni/Ce-MCM-41 catalyst of 1400 min on stream becomes stable as a result of the removal of carbon species from the specific catalyst surface. Then, the removal of carbon causes the active Ni particle to remain clean and stabilized. The lifespan of the catalyst is prolonged.
Urdiana et al. [79] compared several transition metals, such as Ni, Co, Cu, Mn, Fe, Zn, and W, supported by mesoporous silica (SBA-15) in methane decomposition. Among the studied catalysts, Ni/SBA-15 displays an optimal activity in terms of methane conversion and catalytic stability for 13 h on stream (Fig. 2). Further study over Ni catalyst supported over various types of silica materials [ZSM-25, ZSM-400, and amorphous silica (AS)] as supported catalysts in methane decomposition. have performed by Awadallah et al. [49] and the results is shown in Fig. 3. The hydrogen yield and stability of Ni/ZSM-400 are higher than those of Ni/ZSM-25 and Ni/AS during the time on stream due to higher metal dispersion and carbon yield as shown in the TEM and TGA results, respectively. The presence of carbon filaments has contributed to high Ni/ZSM-400 stability. The thicker the carbon filaments are, the higher the catalytic stability will be. In other work, Awadallah et al. [70] evaluated the performance of the Ni catalyst supported by aluminosilicate (Si–Al) and found that the formation of Ni silicate has interrupted the system, resulting in a low catalytic activity with the reaction time. Among all outstanding activities of various supports over the Ni catalyst, the metal oxide support, especially SiO2 and TiO2, possesses an outstanding activity because of their high surface area and thermal stability. The selection of catalyst support and the amount of the Ni catalyst loading on the support also affect the performance of the catalyst in methane decomposition.

adopted from Urdiana et al. [79]
Enhanced stability of Ni/SBA-15 in methane decomposition at a reaction temperature of 500 °C for 13 h on stream. Results are plotted on the basis of methane conversion
Comparison of different silica materials as a catalyst support in terms of hydrogen yield from methane decomposition [49]
Effect of nickel loading
The nickel loading on the catalyst support affects its catalytic activity because it influences the metal–support interaction and dispersion of Ni particles on the support [111]. Corresponding to García-Sancho et al. [50], Salmones et al. [80] found that high Ni loading results in fast deactivation. This finding can be explained by the low availabilities of active Ni particles inside the pores formed by carbon formation, which has enclosed the Ni particles in a short time (Fig. 4). The particle size of Ni increased as the loading of Ni increased from 15 wt% up to 50 wt%. The increase in metallic Ni availability in methane decomposition causes an increase in the yield of MWCNTs, and catalyst deactivation can be inhibited [72]. The accumulation of active Ni species on a supporting catalyst has caused a decrease in the BET surface area of the catalyst [66]. In contrast to the low Ni loading case, most Ni particles are found at the tip of the carbon produced and on the pore opening. Therefore, the availabilities of Ni particles remain high even for a long time on stream.
Effect of loading on catalyst deactivation [80]
Figure 5 shows a TPR graph of the catalyst with different Ni loadings. The results have shown that the reduction temperature of peak III shifts towards a low temperature as the Ni loading increases. This shifting is attributed to the weak Ni-support interaction. As loading increases, the tendency of fast deactivation also increases because of large pores. Saraswat and Pant [94] have studied different loadings (30 wt%, 40 wt%, 50 wt%, 60 wt%, and 70 wt%) of Ni on SiO2 support. The activity of the catalyst in methane decomposition is in the following order: 50% Ni/SiO2 > 40% Ni/SiO2 > 60% Ni/SiO2 > 30% Ni/SiO2 > 70% Ni/SiO2. The decrease in activity after 50% Ni loading may be caused by the increase in particle size, thus lowering the surface area of the catalyst. Furthermore, the low pore density in high Ni catalyst loading may be due to the agglomeration of Ni particles at a high calcination temperature during catalyst preparation. This condition affects the performance of the catalyst in methane decomposition. Uddin et al. [58] evaluated the activity of Ni/Y zeolite catalyst with different Ni loadings of 15 wt% and 30 wt%. The result has shown that 30 wt% Ni catalyst has high initial activity. However, the activity rapidly dropped due to the accumulation of carbon caused by the considerable Ni species on the catalyst surface. The high activity of the catalyst may be due to the synergistic effect of the surface of zeolite and active Ni species. Although many studies have reported that high loading leads to the fast deactivation of the catalyst in methane decomposition due to the increased in the particle size of Ni as reported by Sikander et al. [91], studies have concluded that the effect of Ni loading depends on the type of the catalyst support used in the reaction.
TPR profile of Ni/Mg–O–Al catalyst with A: 15 wt%, B: 25 wt%, and C: 50 wt% of Ni loading [80]
Effect of preparation method
The catalytic activity may be influenced by the preparation method of the catalyst through the dispersion of metal particles on the surface of the catalyst support, which can lead to strong or weak metal–support interactions. Moreover, the reduction rate of Ni and nucleation of metallic Ni may be affected by the preparation method of the catalyst. The redox properties and surface morphology of the catalyst also differ depending on the synthesis condition of the preparation method [112]. In addition to the simplest and common preparation, which is impregnation [103, 106, 112], sol–gel method is commonly applied to synthesize catalysts in methane decomposition because of its simplicity and low cost [62, 63, 74, 82]. This surfactant-assisted technique is used in the sol–gel method because it can improve the access of metal particles to active sites [75]. The role of a surfactant is the same as that of a stabilizer to prevent the accumulation of metal nanoparticles onto metal oxide matrices [115]. This surfactant can control the diameter and shape of metal particles. The catalyst prepared by the impregnation route has a higher degree of crystal agglomeration than that prepared by the sol–gel route, which reduces the interaction effect between metal and support and consequently decreases the reduction temperature of catalysts [103].
Pudukudy et al. [64] used a fusion method to synthesize catalysts. The result has shown that this method is time-saving because the one-pot drying method is used, which does not involve time-consuming steps similar to conventional wet synthesis methods. Lazaro et al. [116] compared the activity of Ni/TiO2 catalyst prepared by impregnation and fusion methods and found that the initial activity of the catalysts prepared via impregnation is higher in the presence of 80 vol% of hydrogen concentration, whereas 60% hydrogen is recorded for the fusion-prepared catalyst. However, the hydrogen concentration (40 vol%) of the impregnation catalyst drastically decreases, whereas the activity of the fusion-prepared catalyst remains stable for approximately 500 min on stream. They concluded that impregnation is less effective in the preparation of this catalyst. Other methods, such as co-precipitation, have been employed in several studies to prepare the catalyst [114, 115]. Guo et al. [119] prepared a Ni-based catalyst supported by mixed-metal oxides and alumina by using conventional impregnation and co-precipitation methods. The catalysts prepared by impregnation produced larger Ni particles than other methods (Fig. 6). Ni particles agglomerate in catalysts produced through impregnation compared with those formed through co-precipitation. The strong interaction between Ni and the support inhibits the formation of large particles caused by the agglomeration of Ni particles. The method chosen to prepare the catalyst has an effect on the dispersion of Ni on the support, which influences the catalytic activity in methane decomposition.
TEM image of NiAl/MMO catalyst prepared through co-precipitation and Ni/Al2O3 fabricated through conventional impregnation [119]
Effect of second metal on a nickel catalyst
The use of a bimetallic catalyst increases the catalytic activity in terms of methane conversion and stability of the catalyst throughout reaction time [81, 84, 85, 94, 101, 103, 105]. Tsoncheva et al. [110] reported that the catalytic activity of methane decomposition increases until it reaches the optimum loading and then decreases when the loading of the active metal continuously increases. The use of bimetallic catalysts and promoters has been introduced to enhance the catalytic activity in methane decomposition, considering the fast deactivation of a Ni catalyst.
The effect of a second metal on a Ni-based catalyst in methane decomposition has been extensively explored. Fe is commonly used as a co-metal in a catalytic reaction [120, 121]. Wang et al. [82] prepared Ni–Fe/SiO2 through the sol–gel method and undiluted methane are decomposed to evaluate the catalytic activity of the catalyst. The incorporation of 40% Fe in 65% Ni/SiO2 has enhanced the stability of the catalyst at higher reaction temperature due to the formation of Ni–Fe alloy as validated in XRD analysis. Bayat et al. [101] investigated the effect of 10% Fe addition on 50% Ni/Al2O3 catalyst. They found that Fe addition has improved the stability of the catalyst by hindering the formation of encapsulating carbon on the catalyst surface, along with an improved rate of carbon diffusion. Despite the improved stability, the catalytic activity of 50% Ni/Al2O3 decreases in the presence of Fe. This finding is attributed to the lower activity of Fe than Ni at a low temperature. The same inference was applied in Ni–Fe supported over calcium silicate catalyst, and the addition of Fe had promoted the formation of carbon nanotubes [122]. The addition of Cu as a second metal on a Ni-based catalyst has been widely considered for methane decomposition [47, 73, 81, 84, 90, 116, 118, 123,124,125,126]. Saraswat and Pant [94] studied the addition of 10% Cu as a promoter in a 50% Ni/SiO2 catalyst. The presence of Cu has increased methane conversion and improved catalytic stability compared with that of the unpromoted catalyst. The effect of Cu may be explained by the Ni–Cu alloy formation, which allows Ni active sites to separate from one another. Therefore, the interactions of adsorbed carbon species, which may lead to the formation of encapsulating carbon, can be minimized [127]. The growth of carbon filaments and the chemisorption of methane are improved upon the introduction of Cu into the system [116]. However, Lua and Wang [128] found that the poor catalytic stability of Ni–Cu alloy is due to the occurrence of a quasi-liquid state, thus leading to the deactivation of a catalyst. Rastegarpanah et al. [44] investigated the effect of various promoters, such as La, Ce, Cu, Co, and Fe, on the catalytic activity of 50 wt% Ni/MgO·Al2O3. Among the studied promoters, 10 wt% Cu added to the catalyst has improved the catalytic activity, hydrogen yield, and methane conversion with the best stability of 10 h on stream. By contrast, the Ce-promoted catalyst showed the highest activity. However, the activity of this catalyst drastically decreases when the temperature increases to higher than 600 °C compared with that of the Cu-promoted catalyst (Fig. 7). The highest stability of Ni–Cu/MgO·Al2O3 can be attributed to the differentiation between the equilibrium rate of carbon formation and its diffusion over active sites. Resistance to coke formation also increases as Cu is incorporated in the catalyst. Guil-López et al. [117] studied the incorporation of Mg and Al in Ni-based catalyst prepared via co-precipitation by the urea method. “Auto regeneration” occurs as temperature increases to 700 °C after the catalyst becomes deactivated at 600 °C. The occurrence of the reversible process is attributed to the reversible change in the mechanism.
Catalytic activity of promoted and unpromoted Ni/MgO·Al2O3 in terms of a methane conversion and b hydrogen yield [44]
Bayat et al. [83] investigated the performance of 50% Ni–Pd/Al2O3 in methane decomposition, which is synthesized by impregnation. The degrees of reducibility and catalytic activity are enhanced upon 15% Pd addition, in agreement with Pudukudy et al. [95]. The rate of carbon diffusion in Pd is higher than that in Ni catalyst. The accumulation of carbon on the metal surface during the reaction can be inhibited by providing a high migration rate of carbon to increase the catalyst's lifespan. Rategarpanah et al. [103] worked on the addition of noble metals (Pd and Pt) as promoters of a 55 wt% Ni–15 wt% Cu/MgO·Al2O3 catalyst prepared via simultaneous sol–gel and wet impregnation methods in the absence of surfactants. The catalytic testing revealed that 4 wt% Pd addition has a more significant effect on catalyst stability during methane decomposition compared to Pt metal. They varied the Pd loading and found that a further increase in loading leads to a slight decrease in the catalytic activity caused by the decrease in the specific surface area and sintering of particles. Chen et al. [69] investigated the effect of ZnO addition on a Ni/Al2O3 catalyst prepared via the co-precipitation method. A ZnAl2O4 spinel-like structure is formed in the interface between ZnO and Al2O3, which can trap NiO particles, thereby making the catalyst highly stable throughout the reaction. The strong interaction between Ni and ZnAl2O4 may hinder the occurrence of the quasi-liquid phenomenon, which causes catalyst deactivation. Gac et al. [51] examined the effect of 38.9 wt% MgO addition on the activity of 26.6 wt% Ni/Al2O3 and showed that the active surface area increases as MgO are introduced to the system. The crystallite size of Ni decreases as a result of strong metal–support interaction and the mechanism of the microstructure arrangement of the catalyst.
Other metal-based catalyst
Noble metals have an outstanding catalytic activity in the catalytic processes [129,130,131,132]. However, the use of these metals as active components is restricted because of their cost. Numerous studies have reported the dissociation mechanism of methane over Pd-based catalyst surfaces in methane decomposition [34, 98, 133, 134]. Szymańska et al. [135] studied the activities of Pt and Pd supported by AC for the production of hydrogen and nanocarbon. The best catalytic activity is achieved by Pd/AC, which shows a higher conversion and stability during the reaction than that by Pt/AC. The presence of deposited carbonaceous species in the form of carbon filaments is a crucial factor that contributes to the high catalyst activity because the blockage of the active sites by the deposited carbon does not occur. The highest activity achieved by Pd/AC catalyst in a prior study with 50 mol% of methane conversion for 4 h on stream at a reaction temperature of 850 °C is attributed to the effectively distributed metallic Pd particles on the AC support [134]. Caballero et al. [113] studied the Rh catalyst supported by γ-Al2O3 prepared through wet impregnation for methane decomposition with the addition of Nd2O3 as a support modifier. The results revealed the methane conversion of the modified catalyst is higher than that of 1 wt% Rh/γ-Al2O3 catalyst attributed to the stronger interaction between Rh and γ-Al2O3–Nd2O3 catalyst support as confirmed through TPR and X-ray photoelectron spectroscopy (XPS) analyses. However, the formation of other byproducts, such as ethane and ethylene, was detected possibly because of the mobility of CHx species on the surface of Rh during methane degradation. Figure 8 shows the mechanism of CHx mobility on the Rh surface for low-loading (a) and high-loading Nd2O3 (b). Notably, no product other than hydrogen has been detected for catalysts with a high Nd2O3 concentration because the CHx species is further adsorbed and highly stable on the Rh surface, thereby generating hydrogen as the only product that results from the aggregation of hydrogen atoms. Caballero et al. found that low amounts of C2H4 and C2H6 are detected with a Pt/γ-Al2O3 catalyst. The incorporation of non-noble metals as promoters is favorable to enhance the catalytic activity of methane decomposition in terms of hydrogen yield, catalyst stability, and controllability of the type of carbon formation, in addition to catalyst cost reduction [85, 95].
Representation of the behavior of methane decomposition on Rh metallic particles for a Rh/Al2O3 and b Rh/Al2O3-10 wt% Nd2O3 catalysts [113]
Majewska and Michalkiewucz [136] reported that high cobalt concentration results in high carbon and hydrogen yields (approximately 95%) at 800 °C in methane decomposition. Co supported by SiO2 displays the best catalytic activity in contrast with other catalyst support such as Al2O3, SiO2, and Nb2O5, for methane decomposition [137]. The catalytic activity increases as the reaction time is prolonged because of the reduction of the remaining cobalt oxide particles that are not reduced during the pre-treatment stage. Zardin and Perez-Lopez [138] studied Co–Al mixed oxides in methane decomposition. The result showed that the low crystallite size enhanced catalytic stability during the reaction. In addition, the presence of oxycarbides also helps hinder the deposition of carbon during the reaction [35]. Ibrahim et al. [24] investigated the effect of Fe loading on the performance of Fe/Al2O3 in methane decomposition. The Fe loading varies between 15 and 100%, and all catalysts have good stability for 240 min on stream without a significant decrease in activity except catalysts with a low Fe loading. In the range of 15% to 100% Fe loading on the catalyst, 60% Fe/Al2O3 exhibits a maximum hydrogen yield of 77.2%. A further increase in Fe loading lowered the catalytic activity due to the aggregation of metallic Fe, thus causing poor Fe dispersion on the support and leading to a low hydrogen yield. The stability test is conducted over 60% Fe/Al2O3. The result showed that no deactivation is observed after 4 h of reaction. The nature of the Fe oxide precursor from which the catalytic active site is generated via reduction strongly influences catalytic stability and hydrogen yields [139]. The introduction of second metals, such as Co, Cu, and Mo, is favorable to the Fe system [62]. The bimetallic catalyst has improved the methane conversion and increased the production of carbon nanotubes (CNTs) caused by the aggregation of metal particles at the high reaction temperature. Cunha et al. [127] explored the effect of 50 wt% Cu addition on 50 wt% Fe-based catalyst and observed the increase in the formation of filamentous carbon and the stability of the catalyst for 5 h on stream. The enhancement of catalyst stability can be attributed to the ensemble control that decreases the possible occurrence of catalyst deactivation. The presence of 15 wt% cobalt in the iron system has increased the catalytic activity of the iron catalyst in hydrogen yield and methane conversion [140]. The existence of appropriate cobalt species and the enhanced dispersion of Co over 30 wt% Fe/Al2O3 catalyst have led to high catalytic activity and stability, with more than 55% of methane conversion for 1400 min on stream. The electronic configuration of Co, and Fe shows an electron deficiency in the 3d orbital that makes methane molecules accessible through partially accepting electrons [141]. A Cu catalyst with a complete 3d orbital produces a low hydrogen yield and generates amorphous carbon as a byproduct in methane decomposition [141]. Although Co-based catalysts exhibit a lower catalytic activity than Ni-based ones do, they have better stability for a longer time on stream in most studies related to methane decomposition [140, 142].
Carbon formation
In addition to hydrogen, carbon is a product of methane decomposition. The obtained carbon may be commercialized for industrial applications. The economics of the process is highly dependent on the quality of carbonaceous products that in turn determine the selling price of carbon. Carbon formation may be in the form of carbon black, CNTs [94, 96], graphite [143], and graphene [62]. The formation of filamentous carbon as a product can contribute to the stability of catalysts [49, 140]. Majewska and Michalkiewucz [136] indicated that a high concentration of metal catalyst (32 wt% Co) produces a high yield of carbon formed at the end of the reaction. Villachampa et al. [144] reported that a further increase in reaction temperature from 500 to 600 °C results in the promotion of carbon filaments and encapsulating carbon. Catalyst deactivation caused by carbon deposition unlikely occurs as long as carbon filaments do not interfere with each other and with the surface of the catalyst support [145]. The deposition of bulk carbon with outstanding physical and chemical properties is one of the benefits associated with methane decomposition. The nanocarbons formed at the end of reaction can also be utilized as electrode materials. As hydrogen yield increases, carbon yield increases. In addition to hydrogen, ordered nanocarbons are formed with metal catalysts, whereas carbonaceous catalysts produce amorphous carbon with different morphologies [88].
Table 2 summarizes the production of carbon from methane decomposition in CNT and graphene forms. Li et al. [146] reported the structure and morphology of the carbon formed are determined by the catalyst and the reaction parameter such as temperature, pressure and the composition of the feedstock used in the reaction. Abdullahi et al. [66] studied Ni/Al2O3 catalyst for methane decomposition and obtained multi-walled CNTs (MWCNTs) as a carbon product and observed that reaction time and temperature influence the carbon yield and graphitization degree of MWCNTs. Meanwhile, Shen and Lua [74] found the formation of carbon filaments over Ni/TiO2 is formed in the form of platelets, hollow tubes, and bow-like and herringbone structures. The herringbone structures of the carbon filaments resulted from the stacking of the graphene layers along the two directions. The variation structure of the filaments was associated to the mechanism of carbon growth on the different catalyst plane and phase. Awadallah et al. [71] reported different SiO2 modifiers in Ni/SiO2 produced graphene sheets and agglomerated Ni particles. The use of metal-based catalysts, such as Ni, Fe, and Cu, which commonly produce a carbon product in a tubular structure, mostly results in MWCNTs or carbon nanofibers, and this type of carbon species contains a graphene layer [91, 92]. Guizani et al. [147] found that a shell-like carbon structure is deposited on a Ni catalyst supported by wood char. At first, carbon is in the form of filamentous and carbon balls as shown in the SEM images (Fig. 9). In addition, the thermal stability of the reaction products, namely, hydrogen gas and carbon, are improved because of the ordered nature of the deposited carbon.
Challenges, recommendations and perspectives
The direct decomposition of methane has become one of the favorable methods in the production of COx-free hydrogen and carbon in various morphologies. The produced carbon can be commercialized and used in practical applications. Moreover, the economy significantly enhances when efficient and low-cost materials and methods in hydrogen production are developed. Among the transition metals studied in methane decomposition, Ni catalyst shows the highest activity in terms of methane conversion and hydrogen yield, together with an enhanced quality of produced carbon. Hence, Ni catalyst is a highly favorable metal because of its high activity in methane decomposition.
Ni catalyst is rapidly deactivated because of the formation of encapsulating carbon covering the active sites of the catalyst and the poor stability of the catalyst at high temperatures. Future research should discover new materials and improve the preparation methods of existing catalysts to obtain highly active and stable Ni catalysts dispersed on catalyst support with a high surface area and better porosity properties. The introduction of promoters on active components and the modification of catalyst support may be beneficial to the enhancement of the stability of catalysts. The addition of promoters, such as CeO2, can help stabilize active sites and facilitate the catalytic activity given that metal oxides are highly common catalyst supports used in methane decomposition. The high surface area of catalyst supports has become one of the critical factors in selecting support for methane decomposition. These properties affect the dispersion of a Ni catalyst, thereby providing enough active sites on the surface of supports. Mesoporous silica materials, such as SBA-15 and MCM-41, are commonly used in methane decomposition. Mesostructured silica nanoparticles (MSNs), which are also a member of the mesoporous silica family, possess a high surface area and a tunable pore size. MSNs have been widely explored in catalysis and commonly used in drug delivery and methanation reaction. Thus, MSNs may become ideal candidates for methane decomposition, which may lead to a high activity because of its high surface area and pore size. Catalyst stability may be improved because MSNs also have high thermal, mechanical, and chemical stability.
In addition to the type of materials used for a reaction, the influence of preparation methods is necessary to determine the catalytic activity because it can generate an improved metal-support interaction, thus enhancing the fine dispersion of Ni particles on the catalyst support. Starting from the simplest method used to prepare the catalysts towards the addition of certain surfactants and also the introduction of the new procedure are investigated in order to develop a catalyst with an enhanced surface structure which then, allowing for a better dissociation of methane on the active sites of the catalyst. The reaction conditions, such as flow rate, temperature, time, and pressure, are critical factors affecting catalytic activities. Therefore, the optimization of the Ni-based catalyst should be explored to achieve optimum conditions.
References
Mandal, R., Kaur, S.: Impact of environmental pollution on trace elements in vegetables and associated potential risk to human health in industrial town Mandi-gobindgarh (India). Chemosphere (2019). https://doi.org/10.1016/j.chemosphere.2018.12.034
Han, X., Sun, T., Feng, Q.: Study on environmental pollution loss measurement model of energy consumption emits and its application in industrial parks. Sci. Total Environ. 668, 1259–1266 (2019). https://doi.org/10.1016/j.scitotenv.2019.03.002
Liang, L., Wang, Z., Li, J.: The effect of urbanization on environmental pollution in rapidly developing urban agglomerations. J. Clean. Prod. 237, 117649 (2019). https://doi.org/10.1016/j.jclepro.2019.117649
Vaidya, P.D., Rodrigues, A.E.: Glycerol reforming for hydrogen production : a review. Chem. Eng. Technol. 32, 1463–1469 (2009). https://doi.org/10.1002/ceat.200900120
Kruger, P.: Alternative Energy Resources: The Quest for Sustainable Energy. Wiley, Hoboken (2006)
Cao, S., Yu, J.: Carbon-based H2-production photocatalytic materials. J. Photochem. Photobiol. C Photochem. Rev. 27, 72–99 (2016)
Wang, Z., Yin, Y., Williams, T., Wang, H., Sun, C., Zhang, X.: Metal link: a strategy to combine graphene and titanium dioxide for enhanced hydrogen production. Int. J. Hydrogen Energy. (2016). https://doi.org/10.1016/j.ijhydene.2016.08.102
Xie, G., Zhang, K., Guo, B., Liu, Q., Fang, L., Gong, J.R.: Graphene-based materials for hydrogen generation from light-driven water splitting. Adv. Mater. (2013). https://doi.org/10.1002/adma.201301207
López Guerra, E., Shanmugharaj, A.M., Choi, W.S., Ryu, S.H.: Thermally reduced graphene oxide-supported nickel catalyst for hydrogen production by propane steam reforming. Appl. Catal. A Gen. 468, 467–474 (2013). https://doi.org/10.1016/j.apcata.2013.09.025
Lu, J., Li, X., He, S., Han, C., Wan, G., Lei, Y., Chen, R., Liu, P., Chen, K., Zhang, L., Luo, Y.: Hydrogen production via methanol steam reforming over Ni-based catalysts: Influences of Lanthanum (La) addition and supports. Int. J. Hydrogen Energy. (2016). https://doi.org/10.1016/j.ijhydene.2016.08.165
Iriondo, A., Barrio, V.L., Cambra, J.F., Arias, P.L., Guemez, M.B., Sanchez-Sanchez, M.C., Navarro, R.M., Fierro, J.L.G.: Glycerol steam reforming over Ni catalysts supported on ceria and ceria-promoted alumina. Int. J. Hydrogen Energy. 35, 11622–11633 (2010). https://doi.org/10.1016/j.ijhydene.2010.05.105
Iriondo, A., Barrio, V.L., Cambra, J.F., Arias, P.L., Güemez, M.B., Navarro, R.M., Sanchez-Sanchez, M.C., Fierro, J.L.G.: Influence of La2O3 modified support and Ni and Pt active phases on glycerol steam reforming to produce hydrogen. Catal. Commun. 10, 1275–1278 (2009). https://doi.org/10.1016/j.catcom.2009.02.004
Seo, J.G., Youn, M.H., Bang, Y., Song, I.K.: Effect of Ni/Al atomic ratio of mesoporous NieAl2O3 aerogel catalysts on their catalytic activity for hydrogen production by steam reforming of liquefied natural gas (LNG). Int. J. Hydrogen Energy. 35, 12174–12181 (2010). https://doi.org/10.1016/j.ijhydene.2010.08.094
Tsoncheva, T., Genova, I., Dimitrov, M., Sarcadi-Priboczki, E., Venezia, A.M., Kovacheva, D., Scotti, N., dal Santo, V.: Nanostructured copper-zirconia composites as catalysts for methanol decomposition. Appl. Catal. B Environ. 165, 599–610 (2015). https://doi.org/10.1016/j.apcatb.2014.10.058
Lorito, D., Paredes-Nunez, A., Mirodatos, C., Schuurman, Y., Meunier, F.C.: Determination of formate decomposition rates and relation to product formation during CO hydrogenation over supported cobalt. Catal. Today. 259, 192–196 (2015). https://doi.org/10.1016/j.cattod.2015.06.027
Tan, H., Li, K., Sioud, S., Cha, D., Amad, M.H., Hedhili, M.N., Al-Talla, Z.A.: Synthesis of Ru nanoparticles confined in magnesium oxide-modified mesoporous alumina and their enhanced catalytic performance during ammonia decomposition. Catal. Commun. 26, 248–252 (2012). https://doi.org/10.1016/j.catcom.2012.06.007
Pinilla, J.L., de Llobet, S., Moliner, R., Suelves, I.: H2-rich gases production from catalytic decomposition of biogas: viability of the process associated to the co-production of carbon nanofibers. Int. J. Hydrogen Energy. 42, 23484–23493 (2017). https://doi.org/10.1016/j.ijhydene.2017.01.119
Tsoncheva, T., Gallo, A., Spassova, I., Dimitrov, M., Genova, I., Marelli, M., Khristova, M., Atanasova, G., Kovacheva, D., dal Santo, V.: Tailored copper nanoparticles in ordered mesoporous KIT-6 silica: preparation and application as catalysts in integrated system for NO removal with products of methanol decomposition. Appl. Catal. A Gen. 464–465, 243–252 (2013). https://doi.org/10.1016/j.apcata.2013.06.006
Abanades, S., Kimura, H., Otsuka, H.: Kinetic investigation of carbon-catalyzed methane decomposition in a thermogravimetric solar reactor. Int. J. Hydrogen Energy. (2015). https://doi.org/10.1016/j.ijhydene.2015.07.023
Schwengber, C.A., Alves, H.J., Schaffner, R.A., Silva, F.A., Sequinel, R., Bach, V.R., Ferracin, R.J.: Overview of glycerol reforming for hydrogen production. Renew. Sustain. Energy Rev. 58, 259–266 (2016). https://doi.org/10.1016/j.rser.2015.12.279
Gallegos-Suárez, E., Guerrero-Ruiz, A., Rodríguez-Ramos, I.: Efficient hydrogen production from glycerol by steam reforming with carbon supported ruthenium catalysts. Carbon N. Y. 96, 578–587 (2016). https://doi.org/10.1016/j.carbon.2015.09.112
Lu, Q., Hou, Y., Laraib, S.R., Khalifa, O., Li, K., Xie, W., Cui, M., Yang, Y.: Electro-catalytic steam reforming of methane over Ni-CeO2/Γ-Al2O3-MgO catalyst. Fuel Process. Technol. 192, 57–64 (2019). https://doi.org/10.1016/j.fuproc.2019.04.021
Iglesias, I., Forti, M., Baronetti, G., Mariño, F.: Zr-enhanced stability of ceria based supports for methane steam reforming at severe reaction conditions. Int. J. Hydrogen Energy. 44, 8121–8132 (2019). https://doi.org/10.1016/j.ijhydene.2019.02.070
Ibrahim, A.A., Fakeeha, A.H., Al-Fatesh, A.S., Abasaeed, A.E., Khan, W.U.: Methane decomposition over iron catalyst for hydrogen production. Int. J. Hydrogen Energy. 40, 7593–7600 (2015). https://doi.org/10.1016/j.ijhydene.2014.10.058
Mondal, K.C., Chandran, S.R.: Evaluation of the economic impact of hydrogen production by methane decomposition with steam reforming of methane process. Int. J. Energy Res. 39, 2–6 (2014). https://doi.org/10.1016/j.ijhydene.2014.04.087
Zhang, J., Li, X., Chen, H., Qi, M., Zhang, G., Hu, H., Ma, X.: Hydrogen production by catalytic methane decomposition: carbon materials as catalysts or catalyst supports. Int. J. Hydrogen Energy. 42, 19755–19775 (2017). https://doi.org/10.1016/j.ijhydene.2017.06.197
Dang, C., Wang, H., Yu, H., Peng, F.: Co–Cu–CaO catalysts for high-purity hydrogen from sorption-enhanced steam reforming of glycerol. Appl. Catal. A Gen. 533, 9–16 (2017). https://doi.org/10.1016/j.apcata.2017.01.006
Abbas, H.F., Daud, W.M.A.: Hydrogen production by methane decomposition: a review. Int. J. Hydrogen Energy. 35, 1160–1190 (2010). https://doi.org/10.1016/j.ijhydene.2009.11.036
Ashik, U.P.M., Daud, W.M.A., Hayashi, J.: A review on methane transformation to hydrogen and nanocarbon: relevance of catalyst characteristics and experimental parameters on yield. Renew. Sustain. Energy Rev. 76, 743–767 (2017). https://doi.org/10.1016/j.rser.2017.03.088
Lopez, E., Kim, J., Shanmugaraj, A.M., Ryu, S.H.: Multiwalled carbon nanotubes-supported nickel catalysts for the steam reforming of propane. J. Mater. Sci. 47, 2985–2994 (2012). https://doi.org/10.1007/s10853-011-6132-1
Nichele, V., Signoretto, M., Menegazzo, F., Gallo, A., Dal, V., Cruciani, G., Cerrato, G.: Glycerol steam reforming for hydrogen production: design of Ni supported catalysts. Appl. Catal. B Environ. 111–112, 225–232 (2012). https://doi.org/10.1016/j.apcatb.2011.10.003
Luo, C., Xie, H., Wang, Q., Luo, G., Liu, C.: A review of the application and performance of carbon nanotubes in fuel cells. J. Nanomater. 2015, 560392 (2015)
Lee, S.C., Seo, H.J., Han, G.Y.: Hydrogen production by catalytic decomposition of methane over carbon black catalyst at high temperatures. Korean J. Chem. Eng. 30, 1716–1721 (2013). https://doi.org/10.1007/s11814-013-0107-7
Kozlov, S.M., Neyman, K.M.: Insights from methane decomposition on nanostructured palladium. J. Catal. 337, 111–121 (2016). https://doi.org/10.1016/j.jcat.2016.02.010
Izhar, S., Kanesugi, H., Tominaga, H., Nagai, M.: Cobalt molybdenum carbides: surface properties and reactivity for methane decomposition. Appl. Catal. A Gen. 317, 82–90 (2007). https://doi.org/10.1016/j.apcata.2006.10.013
Kim, J., Kim, J., Lee, D.: Glycerol steam reforming on Ru catalysts supported on core-shell metal—ceramic microcomposites developed by a microwave-induced hydrothermal method. Appl. Catal. A Gen. 499, 197–204 (2015). https://doi.org/10.1016/j.apcata.2015.04.012
Nishii, H., Miyamoto, D., Umeda, Y., Hamaguchi, H., Suzuki, M., Tanimoto, T., Harigai, T., Takikawa, H., Suda, Y.: Catalytic activity of several carbons with different structures for methane decomposition and by-produced carbons. Appl. Surf. Sci. (2019). https://doi.org/10.1016/j.apsusc.2018.12.073
Papageridis, K.N., Siakavelas, G., Charisiou, N.D., Avraam, D.G., Tzounis, L., Kousi, K., Goula, M.A.: Comparative study of Ni Co, Cu supported on γ-alumina catalysts for hydrogen production via the glycerol steam reforming reaction. Fuel Process. Technol. 152, 156–175 (2016). https://doi.org/10.1016/j.fuproc.2016.06.024
Kumar, A., Chakraborty, J.P., Singh, R.: Bio-oil : the future of hydrogen generation. Biofuels 8, 663–674 (2016). https://doi.org/10.1080/17597269.2016.1141276
Li, Y., Gao, W., Ci, L., Wang, C., Ajayan, P.M.: Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon N. Y. 48, 1124–1130 (2010). https://doi.org/10.1016/j.carbon.2009.11.034
Goula, M.A., Charisiou, N.D., Papageridis, K.N., Delimitis, A., Pachatouridou, E., Iliopoulou, E.F.: Nickel on alumina catalysts for the production of hydrogen rich mixtures via the biogas dry reforming reaction: influence of the synthesis method. Int. J. Hydrogen Energy. 40, 9183–9200 (2015). https://doi.org/10.1016/j.ijhydene.2015.05.129
Weger, L., Abanades, A., Butler, T.: Methane cracking as a bridge technology to the hydrogen economy. Int. J. Hydrogen Energy. 42, 720–731 (2016). https://doi.org/10.1016/j.ijhydene.2016.11.029
Keipi, T., Tolvanen, K.E.S., Tolvanen, H., Konttinen, J.: Thermo-catalytic decomposition of methane: the effect of reaction parameters on process design and the utilization possibilities of the produced carbon. Energy Convers. Manag. 126, 923–934 (2016). https://doi.org/10.1016/j.enconman.2016.08.060
Rastegarpanah, A., Meshkani, F., Rezaei, M.: Thermocatalytic decomposition of methane over mesoporous nanocrystalline promoted Ni/MgO·Al2O3 catalysts. Int. J. Hydrogen Energy. 42, 16476–16488 (2017). https://doi.org/10.1016/j.ijhydene.2017.05.044
Pudukudy, M., Yaakob, Z., Takriff, M.S.: Methane decomposition over unsupported mesoporous nickel ferrites: effect of reaction temperature on the catalytic activity and properties of nanocarbons. RSC Adv. (2016). https://doi.org/10.1039/C6RA14660K
Gonzalez, O.A., Valenzuela, M.A., Wang, J.-A.: Catalytic decomposition of methane over cerium-doped Ni catalysts. In: Material Research Society (2006)
Pinilla, J.L., Suelves, I., Lazaro, M.J., Moliner, R., Palacios, J.M.: Activity of NiCuAl catalyst in methane decomposition studied using a thermobalance and the structural changes in the Ni and the deposited carbon. Int. J. Hydrogen Energy. 33, 2515–2524 (2008). https://doi.org/10.1016/j.ijhydene.2008.02.041
Wang, Z., Navarrete, J.: Production of carbon nanotubes and hydrogen catalyzed with Ni/MCM-41 catalysts. Green Sustain. Chem. 2, 91–96 (2012)
Awadallah, A.E., El-Desouki, D.S., Aboul-Gheit, N.A.K., Ibrahim, A.H., Aboul-Gheit, A.K.: Effect of crystalline structure and pore geometry of silica based supported materials on the catalytic behavior of metallic nickel particles during methane decomposition to COx-free hydrogen and carbon nanomaterials. Int. J. Hydrogen Energy. 1, 1–13 (2016). https://doi.org/10.1016/j.ijhydene.2016.07.081
García-Sancho, C., Guil-López, R., Pascual, L., Maireles-Torres, P., Navarro, R.M., Fierro, J.L.G.: Optimization of nickel loading of mixed oxide catalyst ex-hydrotalcite for H2 production by methane decomposition. Appl. Catal. A Gen. (2017). https://doi.org/10.1016/j.apcata.2017.07.038
Gac, W., Denis, A., Borowiecki, T., Kepiński, L.: Methane decomposition over Ni–MgO–Al2O3 catalysts. Appl. Catal. A Gen. 357, 236–243 (2009). https://doi.org/10.1016/j.apcata.2009.01.029
Fakeeha, A.H., Khan, W.U., Al-Fatesh, A.S., Abasaeed, A.E., Naeem, M.A.: Production of hydrogen and carbon nanofibers from methane over Ni–Co–Al catalysts. Int. J. Hydrogen Energy. 1, 4–11 (2014). https://doi.org/10.1016/j.ijhydene.2014.12.011
Bai, Z., Chen, H., Li, B., Li, W.: Methane decomposition over Ni loaded activated carbon for hydrogen production and the formation of filamentous carbon. Int. J. Hydrogen Energy. 32, 32–37 (2007). https://doi.org/10.1016/j.ijhydene.2006.06.030
Ashik, U.P.M., Daud, W.M.A.W.: Stabilization of Ni, Fe and Co nanoparticles through modified Stöber method to obtain excellent catalytic performance: preparation, characterization, and catalytic activity for methane decomposition. J. Taiwan Inst. Chem. Eng. 1, 1–14 (2016). https://doi.org/10.1016/j.jtice.2015.12.019
Ying, Y., Meisheng, C., Minglai, L., Na, Z., Zhiqi, L., Yongxi, S.: Rare earth modified Ni–Si catalysts for hydrogen production from methane decomposition. J. Rare Earths. 32, 709–714 (2014). https://doi.org/10.1016/S1002-0721(14)60130-7
Zhang, J., Jin, L., Li, Y., Hu, H.: Ni doped carbons for hydrogen production by catalytic methane decomposition. Int. J. Hydrogen Energy. 38, 3937–3947 (2013). https://doi.org/10.1016/j.ijhydene.2013.01.105
Uddin, M.N., Daud, W.M.A., Abbas, H.F.: Kinetics and deactivation mechanisms of the thermal decomposition of methane in hydrogen and carbon nanofiber Co-production over Ni-supported Y zeolite-based catalysts. Energy Convers. Manag. 87, 796–809 (2014). https://doi.org/10.1016/j.enconman.2014.07.072
Uddin, M.N., Daud, W.M.A., Abbas, H.F.: Co-production of hydrogen and carbon nanofibers from methane decomposition over zeolite Y supported Ni catalysts. Energy Convers. Manag. 90, 218–229 (2015). https://doi.org/10.1016/j.enconman.2014.10.060
Lua, A.C., Wang, H.Y.: Decomposition of methane over unsupported porous nickel and alloy catalyst. Appl. Catal. B Environ. 132–133, 469–478 (2013). https://doi.org/10.1016/j.apcatb.2012.12.014
Guil-Lopez, R., Botas, J.A., Fierro, J.L.G., Serrano, D.P.: Comparison of metal and carbon catalysts for hydrogen production by methane decomposition. Appl. Catal. A Gen. 396, 40–51 (2011). https://doi.org/10.1016/j.apcata.2011.01.036
Maneerung, T., Hidajat, K., Kawi, S.: Co-production of hydrogen and carbon nanofibers from catalytic decomposition of methane over LaNi(1–x)MxO3-a perovskite (where M = Co, Fe and X = 0, 0.2, 0.5, 0.8, 1). Int. J. Hydrogen Energy. 40, 13399–13411 (2015). https://doi.org/10.1016/j.ijhydene.2015.08.045
Pudukudy, M., Yaakob, Z., Mazuki, M.Z., Takriff, M.S., Jahaya, S.S.: One-pot sol–gel synthesis of MgO nanoparticles supported nickel and iron catalysts for undiluted methane decomposition into COx free hydrogen and nanocarbon. Appl. Catal. B Environ. (2017). https://doi.org/10.1016/j.apcatb.2017.04.070
Pudukudy, M., Yaakob, Z., Kadier, A., Takriff, M.S., Mat Hassan, N.S.: One-pot sol–gel synthesis of Ni/TiO2 catalysts for methane decomposition into COx free hydrogen and multiwalled carbon nanotubes. Int. J. Hydrogen Energy. 1, 1–19 (2017). https://doi.org/10.1016/j.ijhydene.2017.04.223
Pudukudy, M., Yaakob, Z., Takrif, M.S.: Methane decomposition into COx free hydrogen and multiwalled carbon nanotubes over ceria, zirconia and lanthana supported nickel catalysts prepared via a facile solid state citrate fusion method. Energy Convers. Manag. 126, 302–315 (2016). https://doi.org/10.1016/j.enconman.2016.08.006
Chesnokov, V.V., Chichkan, A.S.: Production of hydrogen by methane catalytic decomposition over Ni–Cu–Fe/Al2O3 catalyst. Int. J. Hydrogen Energy. 34, 2979–2985 (2009). https://doi.org/10.1016/j.ijhydene.2009.01.074
Abdullahi, I., Sakulchaicharoen, N., Herrera, J.E.: A mechanistic study on the growth of multi-walled carbon nanotubes by methane decomposition over nickel-alumina catalyst. Diam. Relat. Mater. 23, 76–82 (2012). https://doi.org/10.1016/j.diamond.2012.01.017
Frusteri, F., Italiano, G., Espro, C., Cannilla, C., Bonura, G.: H2 production by methane decomposition: catalytic and technological aspects. Int. J. Hydrogen Energy. 37, 16367–16374 (2012). https://doi.org/10.1016/j.ijhydene.2012.02.192
Wen, Y., Wei, C., Chengfa, J., Jie, W., Wenjing, S.: Cerium oxide promoted Ni/MgO catalyst for the synthesis of multi-walled carbon nanotubes. Chin. J. Catal. 32, 1323–1328 (2011). https://doi.org/10.1016/S1872-2067(10)60247-1
Chen, J., Qiao, Y., Li, Y.: Modification of Ni state to promote the stability of Ni–Al2O3 catalyst in methane decomposition to produce hydrogen and carbon nanofibers. J. Solid State Chem. 191, 107–113 (2012). https://doi.org/10.1016/j.jssc.2012.03.019
Awadallah, A.E., Ahmed, W., Noor El-Din, M.R., Aboul-Enein, A.A.: Novel aluminosilicate hollow sphere as a catalyst support for methane decomposition to COx-free hydrogen production. Appl. Surf. Sci. 287, 415–422 (2013). https://doi.org/10.1016/j.apsusc.2013.09.173
Awadallah, A.E., Solyman, S.M., Aboul-Enein, A.A., Ahmed, H.A., Aboul-Gheit, N.A.K., Hassan, S.A.: Effect of combining Al, Mg, Ce or La oxides to extracted rice husk nanosilica on the catalytic performance of NiO during COx-free hydrogen production via methane decomposition. Int. J. Hydrogen Energy. (2017). https://doi.org/10.1016/j.ijhydene.2017.03.049
Awadallah, A.E., Mostafa, M.S., Aboul-Enein, A.A., Hanafi, S.A.: Hydrogen production via methane decomposition over Al2O3–TiO2 binary oxides supported Ni catalysts: effect of Ti content on the catalytic efficiency. Fuel 129, 68–77 (2014). https://doi.org/10.1016/j.fuel.2014.03.047
Pinilla, J.L., Suelves, I., Lázaro, M.J., Moliner, R., Palacios, J.M.: Parametric study of the decomposition of methane using a NiCu/Al2O3 catalyst in a fluidized bed reactor. Int. J. Hydrogen Energy. 35, 9801–9809 (2010). https://doi.org/10.1016/j.ijhydene.2009.10.008
Shen, Y., Lua, A.C.: Sol-gel synthesis of titanium oxide supported nickel catalysts for hydrogen and carbon production by methane decomposition. J. Power Sources. 280, 467–475 (2015). https://doi.org/10.1016/j.jpowsour.2015.01.057
Tapia-Parada, K., Valverde-Aguilar, G., Mantilla, A., Valenzuela, M.A., Hernandez, E.: Synthesis and characterization of Ni/Ce–SiO2 and Co/Ce–TiO catalysts for methane decomposition. Fuel 110, 70–75 (2013). https://doi.org/10.1016/j.fuel.2012.11.022
Nuernberg, G.D.B., Foletto, E.L., Campos, C.E.M., Fajardo, H.V., Carreno, N.L.V., Probst, L.F.D.: Direct decomposition of methane over Ni catalyst supported in magnesium aluminate. J. Power Sources. 208, 409–414 (2012). https://doi.org/10.1016/j.jpowsour.2012.02.037
Tanggarnjanavalukul, C., Donphai, W., Witoon, T., Chareonpanich, M., Limtrakul, J.: Deactivation of nickel catalysts in methane cracking reaction: effect of bimodal meso–macropore structure of silica support. Chem. Eng. J. 262, 364–371 (2015). https://doi.org/10.1016/j.cej.2014.09.112
Guevara, J.C., Wang, J.A., Chen, L.F., Valenzuela, M.A., Salas, P., García-Ruiz, A., Toledo, J.A., Cortes-Jácome, M.A., Angeles-Chavez, C., Novaro, O.: Ni/Ce-MCM-41 mesostructured catalysts for simultaneous production of hydrogen and nanocarbon via methane decomposition. Int. J. Hydrogen Energy. 35, 3509–3521 (2010). https://doi.org/10.1016/j.ijhydene.2010.01.068
Urdiana, G., Valdez, R., Lastra, G., Valenzuela, M., Olivas, A.: Production of hydrogen and carbon nanomaterials using transition metal catalysts through methane decomposition. Mater. Lett. 217, 9–12 (2018). https://doi.org/10.1016/j.matlet.2018.01.033
Salmones, J., Wang, J.A., Valenzuela, M.A., Sanchez, E., Garcia, A.: Pore geometry influence on the deactivation behavior of Ni-based catalysts for simultaneous production of hydrogen and nanocarbon. Catal. Today. 148, 134–139 (2009). https://doi.org/10.1016/j.cattod.2009.03.005
Li, J., Zhao, L., He, J., Dong, L., Xiong, L., Du, Y., Yang, Y., Wang, H., Peng, S.: Methane decomposition over high-loaded Ni–Cu–SiO2 catalysts. Fusion Eng. Des. (2016). https://doi.org/10.1016/j.fusengdes.2016.06.046
Wang, W., Wang, H., Yang, Y., Jiang, S.: Ni–SiO2 and Ni–Fe–SiO2 catalysts for methane decomposition to prepare hydrogen and carbon filaments. Int. J. Hydrogen Energy. 37, 2–10 (2012). https://doi.org/10.1016/j.ijhydene.2012.03.003
Bayat, N., Rezaei, M., Meshkani, F.: Hydrogen and carbon nanofibers synthesis by methane decomposition over Ni-Pd/Al2O3 catalyst. Int. J. Hydrogen Energy. (2016). https://doi.org/10.1016/j.ijhydene.2016.01.134
Li, J., Gong, Y., Chen, C., Hou, J., Yue, L., Fu, X., Zhao, L., Chen, H., Wang, H., Peng, S.: Evolution of the Ni–Cu–SiO2 catalyst for methane decomposition to prepare hydrogen. Fusion Eng. Des. (2017). https://doi.org/10.1016/j.fusengdes.2017.05.040
Pudukudy, M., Yaakob, Z., Takriff, M.S.: Methane decomposition over Pd promoted Ni/MgAl2O4 catalyst for the production of COx free hydrogen and multiwalled carbon nanotubes. Appl. Surf. Sci. (2015). https://doi.org/10.1016/j.apsusc.2015.08.246
Pudukudy, M., Kadier, A., Yaakob, Z., Takriff, M.S.: Non-oxidative thermocatalytic decomposition of methane into COx free hydrogen and nanocarbon over unsupported porous NiO and Fe2O3 catalysts. Int. J. Hydrogen Energy. 1, 1–13 (2016). https://doi.org/10.1016/j.ijhydene.2016.08.160
Zhang, W., Ge, Q., Xu, H.: Influences of reaction conditions on methane decomposition over non-supported Ni catalyst. J. Nat. Gas Chem. 20, 339–344 (2011). https://doi.org/10.1016/S1003-9953(10)60205-8
Srilatha, K., Bhagawan, D., Kumar, S.S., Himabindu, V.: Sustainable fuel production by thermocatalytic decomposition of methane—a review. S. Afr. J. Chem. Eng. (2017). https://doi.org/10.1016/j.sajce.2017.10.002
Serrano, D.P., Botas, J.A., Fierro, J.L.G., Guil-López, R., Pizarro, P., Gómez, G.: Hydrogen production by methane decomposition: origin of the catalytic activity of carbon materials. Fuel 89, 1241–1248 (2010). https://doi.org/10.1016/j.fuel.2009.11.030
Cazaña, F., Latorre, N., Tarifa, P., Labarta, J., Romeo, E., Monzón, A.: Synthesis of graphenic nanomaterials by decomposition of methane on a Ni–Cu/biomorphic carbon catalyst. Kinetic and characterization results. Catal. Today. (2017). https://doi.org/10.1016/j.cattod.2017.03.056
Sikander, U., Samsudin, M.F., Sufian, S., KuShaari, K., Kait, C.F., Naqvi, S.R., Chen, W.-H.: Tailored hydrotalcite-based Mg–Ni–Al catalyst for hydrogen production via methane decomposition: effect of nickel concentration and spinel-like structures. Int. J. Hydrogen Energy. 44, 14424–14433 (2019). https://doi.org/10.1016/j.ijhydene.2018.10.224
Pinilla, J.L., Torres, D., Lazaro, M.J., Suelves, I., Moliner, R., Cannadas, I., Rodrıguez, J., Vidal, A., Martınez, D.: Metallic and carbonaceous -based catalysts performance in the solar catalytic decomposition of methane for hydrogen and carbon production. Int. J. Hydrogen Energy. 37, 9645–9655 (2012). https://doi.org/10.1016/j.ijhydene.2012.03.075
Pudukudy, M., Yaakob, Z.: Methane decomposition over Ni, Co and Fe based monometallic catalysts supported on sol gel derived SiO2 microflakes. Chem. Eng. J. 262, 1009–1021 (2015). https://doi.org/10.1016/j.cej.2014.10.077
Saraswat, S.K., Pant, K.K.: Synthesis of hydrogen and carbon nanotubes over copper promoted Ni/SiO2 catalyst by thermocatalytic decomposition of methane. J. Nat. Gas Sci. Eng. 13, 52–59 (2013). https://doi.org/10.1016/j.jngse.2013.04.001
Pudukudy, M., Yaakob, Z., Akmal, Z.S.: Direct decomposition of methane over Pd promoted Ni/SBA-15 catalyst. Appl. Surf. Sci. (2015). https://doi.org/10.1016/j.apsusc.2015.06.073
Saraswat, S.K., Pant, K.K.: Synthesis of carbon nanotubes by thermo catalytic decomposition of methane over Cu and Zn promoted Ni/MCM-22 catalyst. J. Environ. Chem. Eng. 1, 746–754 (2013). https://doi.org/10.1016/j.jece.2013.07.009
Figueiredo, J.L., Orfao, J.J.M., Cunha, A.F.: Hydrogen production via methane decomposition on Raney-type catalysts. Int. J. Hydrogen Energy. 35, 9795–9800 (2010). https://doi.org/10.1016/j.ijhydene.2009.12.071
Salam, M.A., Abdullah, B.: Catalysis mechanism of Pd-promoted γ-alumina in the thermal decomposition of methane to hydrogen: a density functional theory study. Mater. Chem. Phys. 188, 18–23 (2017). https://doi.org/10.1016/j.matchemphys.2016.12.022
Dou, B., Wang, C., Song, Y., Chen, H., Xu, Y.: Activity of Ni–Cu–Al based catalyst for renewable hydrogen production from steam reforming of glycerol. Energy Convers. Manag. 78, 253–259 (2014). https://doi.org/10.1016/j.enconman.2013.10.067
Gupta, R.B.: Hydrogen fuel: production, transport and storage. CRC Press, Boca Raton (2009)
Bayat, N., Rezaei, M., Meshkani, F.: Methane decomposition over Ni-Fe/Al2O3 catalysts for production of COx-free hydrogen and carbon nanofiber. Int. J. Hydrogen Energy. 41, 1574–1584 (2016). https://doi.org/10.1016/j.ijhydene.2015.10.053
Contreras, J.L., Salmones, J., Colin-Luna, J.A., Nuno, L., Quintana, B., Cordova, I., Zeifert, B., Tapia, C., Fuentes, G.A.: Catalysts for H2 production using the ethanol steam reforming ( a review ). Int. J. Hydrogen Energy. 39, 18835–18853 (2014). https://doi.org/10.1016/j.ijhydene.2014.08.072
Rategarpanah, A., Meshkani, F., Wang, Y., Arandiyan, H., Rezaei, M.: Thermocatalytic conversion of methane to highly pure hydrogen over Ni–Cu/MgO·Al2O3 catalysts: Influence of noble metals (Pt and Pd) on the catalytic activity and stability. Energy Convers. Manag. 166, 268–280 (2018). https://doi.org/10.1016/j.enconman.2018.04.033
Tian, M., Li, K., Zhu, X., Wei, Y., Zheng, Y., Zhang, L., Long, Y., Wang, H.: Modified Al@Al2O3 phase change materials by carbon via in-situ catalytic decomposition of methane. Sol. Energy Mater. Sol. Cells. 200, 109924 (2019). https://doi.org/10.1016/j.solmat.2019.109924
Rastegarpanah, A., Rezaei, M., Meshkani, F., Zhang, K., Zhao, X., Pei, W., Liu, Y., Deng, J., Arandiyan, H., Dai, H.: Influence of group VIB metals on activity of the Ni/MgO catalyst for methane decomposition. Appl. Catal. B Environ. (2019). https://doi.org/10.1016/j.apcatb.2019.01.067
Wang, D., Zhang, J., Sun, J., Gao, W., Cui, Y.: Effect of metal additives on the catalytic performance of Ni/Al2O3 catalyst in thermocatalytic decomposition of methane. Int. J. Hydrogen Energy. (2019). https://doi.org/10.1016/j.ijhydene.2019.01.272
Najafishirtari, S., Guglieri, C., Marras, S., Scarpellini, A., Brescia, R., Prato, M., Righi, G., Franchini, A., Magri, R., Manna, L., Colombo, M.: Metal-support interaction in catalysis: the influence of the morphology of a nano-oxide domain on catalytic activity. Appl. Catal. B Environ. (2018). https://doi.org/10.1016/j.apcatb.2018.06.033
Lai, C.: Mesoporous silica nanomaterials applications in catalysis. J. Thermodyn. Catal. 5, 1–3 (2013). https://doi.org/10.4172/2157-7544.1000e124
Palacio, R., Gallego, J., Gabelica, Z., Batiot-Dupeyrat, C., Barrault, J., Valange, S.: Decomposition of ethanol into H2-rich gas and carbon nanotubes over Ni, Co and Fe supported on SBA-15 and Aerosil. Appl. Catal. A Gen. (2015). https://doi.org/10.1016/j.apcata.2015.03.041
Tsoncheva, T., Genova, I., Stoyanova, M., Pohl, M.-M., Nickolov, R., Dimitrov, M., Sarcadi-Priboczki, E., Mihaylov, M., Kovacheva, D., Hadjiivanov, K.: Effect of mesoporous silica topology on the formation of active sites in copper supported catalysts for methanol decomposition. Appl. Catal. B Environ. 147, 684–697 (2014). https://doi.org/10.1016/j.apcatb.2013.10.002
Aziz, M.A.A., Jalil, A.A., Triwahyono, S., Ahmad, A.: CO2 methanation over heterogenous catalyst: recent progress and future prospects. R. Soc. Chem. (2015)
Jana, P., de la Pena O’Shea, V.A., Coronado, J.M., Serrano, D.P.: Cobalt based catalysts prepared by Pechini method for CO2-free hydrogen production by methane decomposition. Int. J. Hydrogen Energy. 35, 3–12 (2010). https://doi.org/10.1016/j.ijhydene.2010.07.125
Caballero, M., Angel, G.D., Bonilla-Sanchez, A., Rangel-Vazquez, I., Arrieta, A., Vazquez-Zavala, A., Huerta, L., Salgado, M.: High selectivity to hydrogen on the methane decomposition over Rh/γ–Al2O3–Nd2O3 catalysts. Int. J. Hydrogen Energy. 1, 1–13 (2016). https://doi.org/10.1016/j.ijhydene.2016.10.001
Caballero, M., Angel, G.D., Rangel-Vazquez, I., Huerta, L.: Hydrogen production by methane decomposition on Pt/γ-alumina doped with neodynium catalyst and its kinetic study. Catal. Today. (2018). https://doi.org/10.1016/j.cattod.2018.05.024
Liz-Marzan, L.M., Lado-Tourino, I.: Reduction and stabilization of silver nanoparticles in ethanol by nonionic surfactants. Langmuir 12, 3585–3589 (1996)
Lazaro, M.J., Echegoyen, Y., Alegre, C., Suelves, I., Moliner, R., Palacios, J.M.: TiO2 as textural promoter on high loaded Ni catalysts for methane decomposition. Int. J. Hydrogen Energy. 33, 3320–3329 (2008). https://doi.org/10.1016/j.ijhydene.2008.03.050
Guil-López, R., La Parola, V., Pena, M.A., Fierro, J.L.G.: Evolution of the Ni-active centres into ex hydrotalcite oxide catalysts during the COx-free hydrogen production by methane decomposition. Int. J. Hydrogen Energy. (2012). https://doi.org/10.1016/j.ijhydene.2011.11.083
Li, D., Chen, J., Li, Y.: Evidence of composition deviation of metal particles of a Ni–Cu/Al2O3 catalyst during methane decomposition to COx-free hydrogen. Int. J. Hydrogen Energy. 34, 299–307 (2009). https://doi.org/10.1016/j.ijhydene.2008.09.106
Guo, Z., Zheng, J.E., Liu, Y., Chu, W.: Insight into the role of metal/oxide interaction and Ni availabilities on NiAl mixed oxide catalyst for methane decomposition. Appl. Catal. A Gen. (2018). https://doi.org/10.1016/j.apcata.2018.01.031
Jiao, Y., Zhang, J., Du, Y., Sun, D., Wang, J., Chen, Y., Lu, J.: Steam reforming of hydrocarbon fuels over M (Fe Co, Ni, Cu, Zn)-Ce bimetal catalysts supported on Al2O3. Int. J. Hydrogen Energy. 41, 10473–10482 (2015). https://doi.org/10.1016/j.ijhydene.2015.09.151
Li, L., Meng, Q., Ji, W., Shao, J., Xu, Q., Yan, J.: Embedded iron nanoparticles by graphitized carbon as highly active yet stable catalyst for ammonia decomposition. Mol. Catal. 442, 147–153 (2017). https://doi.org/10.1016/j.mcat.2017.09.013
Tezel, E., Figen, H.E., Baykara, S.Z.: Hydrogen production by methane decomposition using bimetallic Ni–Fe catalysts. Int. J. Hydrogen Energy. 44, 9930–9940 (2019). https://doi.org/10.1016/j.ijhydene.2018.12.151
Shen, Y., Lua, A.C.: Polyol synthesis of nickel-copper based catalysts for hydrogen production by methane decomposition. Int. J. Hydrogen Energy. 1, 1–11 (2014). https://doi.org/10.1016/j.ijhydene.2014.10.071
Wolfbeisser, A., Kovács, G., Kozlov, S.M., Föttinger, K., Bernardi, J., Klötzer, B., Neyman, K.M., Rupprechter, G.: Surface composition of CuNi–ZrO2 during methane decomposition: an operando NAP-XPS and density functional study. Catal. Today. 1, 2–11 (2016). https://doi.org/10.1016/j.cattod.2016.04.022
Borghei, M., Karimzadeh, R., Rashidi, A., Izadi, N.: Kinetics of methane decomposition to COx-free hydrogen and carbon nanofiber over Ni–Cu/MgO catalyst. Int. J. Hydrogen Energy. 35, 9479–9488 (2010). https://doi.org/10.1016/j.ijhydene.2010.05.072
Hornés, A., Bera, P., Fernández-García, M., Guerrero-Ruiz, A., Martínez-Arias, A.: Catalytic and redox properties of bimetallic Cu–Ni systems combined with CeO2 or Gd-doped CeO2 for methane oxidation and decomposition. Appl. Catal. B Environ. 111–112, 96–105 (2012). https://doi.org/10.1016/j.apcatb.2011.09.022
Cunha, A.F., Órfão, J.J.M., Figueiredo, J.L.: Methane decomposition on Fe–Cu Raney-type catalysts. Fuel Process. Technol. 90, 1234–1240 (2009). https://doi.org/10.1016/j.fuproc.2009.06.004
Lua, A.C., Wang, H.Y.: Hydrogen production by catalytic decomposition of methane over Ni–Cu–Co alloy particles. Appl. Catal. B Environ. 156–157, 84–93 (2014). https://doi.org/10.1016/j.apcatb.2014.02.046
Pan, Y., Wen, M.: Noble metals enhanced catalytic activity of anatase TiO2 for hydrogen evolution reaction. Int. J. Hydrogen Energy. 43, 22055–22063 (2018). https://doi.org/10.1016/j.ijhydene.2018.10.093
Yadav, A.K., Vaidya, P.D.: A study on the efficacy of noble metal catalysts for butanol steam reforming. Int. J. Hydrogen Energy. 44, 25575–25588 (2019). https://doi.org/10.1016/j.ijhydene.2019.07.191
Santos, J.L., Mäki-Arvela, P., Wärnå, J., Monzón, A., Centeno, M.A., Murzin, D.Y.: Hydrodeoxygenation of vanillin over noble metal catalyst supported on biochars: part II: catalytic behaviour. Appl. Catal. B Environ. (2019). https://doi.org/10.1016/j.apcatb.2019.118425
Korobeinyk, A.V., Whitby, R.D.L., Mikhalovsky, S.V., Inglezakis, V.J.: In situ production of high purity noble metal nanoparticles on fumed silica and catalytic activity towards 2-nitrophenol reduction. J. Phys. Chem. Solids. 127, 28–34 (2019). https://doi.org/10.1016/j.jpcs.2018.12.001
Reyhani, A., Mortazavi, S.Z., Parvin, P., Mahmoudi, Z.: Simultaneous laser induced breakdown spectroscopy and Pd-assisted methane decomposition at different pressures. Spectrochim. Acta Part B. 74–75, 124–130 (2012). https://doi.org/10.1016/j.sab.2012.06.050
Sarada Prasad, J., Dhand, V., Himabindu, V., Anjaneyulu, Y., Jain, P.K.: Production of hydrogen and carbon nanofibers through the decomposition of methane over activated carbon supported Pd catalysts. Int. J. Hydrogen Energy. 35, 10977–10983 (2010). https://doi.org/10.1016/j.ijhydene.2010.07.021
Szymanska, M., Malaika, A., Rechnia, P., Miklaszewska, A., Kozłowski, M.: Metal/activated carbon systems as catalysts of methane decomposition reaction. Catal. Today. 249, 94–102 (2015). https://doi.org/10.1016/j.cattod.2014.11.025
Majewska, J., Michalkiewicz, B.: Production of hydrogen and carbon nanomaterials from methane using Co/ZSM-5 catalyst. Int. J. Hydrogen Energy. 41, 8668–8678 (2016). https://doi.org/10.1016/j.ijhydene.2016.01.097
Silva, R.R.C.M., Oliveira, H.A., Guarino, A.C.P.F., Toledo, B.B., Moura, M.B.T., Oliveira, T.M., Passos, F.B.: Effect of support on methane decomposition for hydrogen production over cobalt catalysts. Int. J. Hydrogen Energy. 41, 6763–6772 (2016)
Zardin, L., Perez-Lopez, O.W.: Hydrogen production by methane decomposition over Co-Al mixed oxides derived from hydrotalcites: Effect of the catalyst activation with. Int. J. Hydrogen Energy. 42, 7895–7907 (2017). https://doi.org/10.1016/j.ijhydene.2017.02.153
Fakeeha, A.H., Ibrahim, A.A., Khan, W.U., Seshan, K., Al Otaibi, R.L., Al-Fatesh, A.S.: Hydrogen production via catalytic methane decomposition over alumina supported iron catalyst. Arab. J. Chem. (2016). https://doi.org/10.1016/j.arabjc.2016.06.012
Al-Fatesh, A.S., Fakeeha, A.H., Khan, W.U., Ibrahim, A.A., He, S., Seshan, K.: Production of hydrogen by catalytic methane decomposition over alumina supported mono-, bi- and tri-metallic catalysts. Int. J. Hydrogen Energy. 41, 22932–22940 (2016). https://doi.org/10.1016/j.ijhydene.2016.09.027
Dupuis, A.-C.: The catalyst in the CCVD of carbon nanotubes—a review. Prog. Mater. Sci. 50, 929–961 (2005). https://doi.org/10.1016/j.pmatsci.2005.04.003
Pudukudy, M., Yaakob, Z., Akmal, Z.S.: Direct decomposition of methane over SBA-15 supported Ni, Co and Fe based bimetallic catalysts. Appl. Surf. Sci. 330, 418–430 (2015). https://doi.org/10.1016/j.apsusc.2015.01.032
Chen, J., Yang, X., Li, Y.: Investigation on the structure and the oxidation activity of the solid carbon produced from catalytic decomposition of methane. Fuel 89, 943–948 (2010). https://doi.org/10.1016/j.fuel.2009.08.017
Villacampa, J.I., Royo, C., Romeo, E., Montoya, J.A., Angel, P.D., Monzón, A.: Catalytic decomposition of methane over Ni-Al2O3 coprecipitated catalysts reaction and regeneration studies. Appl. Catal. A Gen. 252, 363–383 (2003). https://doi.org/10.1016/S0926-860X(03)00492-7
Zhang, T., Amiridis, M.D.: Hydrogen production via the direct cracking of methane over silica-supported nickel catalysts. Appl. Catal. A Gen. 167, 161–172 (1998)
Li, Y., Li, D., Wang, G.: Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: a review. Catal. Today. 162, 1–48 (2011). https://doi.org/10.1016/j.cattod.2010.12.042
Guizani, C., Sanz, F.J.E., Salvador, S.: The nature of the deposited carbon at methane cracking over a nickel loaded wood-char. Comptes Rendus Chim. 19, 423–432 (2016). https://doi.org/10.1016/j.crci.2015.10.009
Naresh, G., Vijay Kumar, V., Anjaneyulu, C., Tardio, J., Bhargava, S.K., Patel, J., Venugopal, A.: Nano size Hβ zeolite as an effective support for Ni and Ni–Cu for COx free hydrogen production by catalytic decomposition of methane. Int. J. Hydrogen Energy. (2016). https://doi.org/10.1016/j.ijhydene.2016.09.131
Acknowledgements
The authors wish to thank the members of Fuel Processing Lab, Fuel Cell Institute, Universiti Kebangsaan Malaysia for their assistance. This work is supported by Universiti Kebangsaan Malaysia under grant numbers (GUP-2017-006) and Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS/1/2019/STG01/UKM/031).
Funding
Universiti Kebangsaan Malaysia under grant numbers (GUP-2017-006) and Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS/1/2019/STG01/UKM/031).
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript. These authors contributed equally.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hasnan, N.S.N., Timmiati, S.N., Lim, K.L. et al. Recent developments in methane decomposition over heterogeneous catalysts: an overview. Mater Renew Sustain Energy 9, 8 (2020). https://doi.org/10.1007/s40243-020-00167-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40243-020-00167-5