Abstract
A series of non-substituted 1,3,5-triaryl-2-pyrazolines and pyrazolines substituted with Fluoro (-F), Chloro (-Cl) and Bromo (-Br) groups at the 3-aryl position were studied. All calculations were done using the conceptual framework of density functional theory. The geometries and reactivity properties were analyzed according to an increase from one to twelve alkyl units in the 5-aryl of 2-pyrazoline ring. In order to be able to apply the particular methodology named KID procedure (for Koopmans in DFT), the KID descriptors were calculated and the results showed that the use of this approximation (Koopmans’ theorem in DFT studies) is feasible. The results for the geometries determined that the increase of the chain with alkyl units does not affect the geometry of the systems. However, the solvation energy also calculated is affected by this increase in the allyl chain length. Due to this, as the chains increases, the solubility of the molecular systems diminishes. The chemical reactivity properties were determined by calculating the descriptors that arise from conceptual DFT and it could be demonstrated that they are not affected by the chain growth. Slight differences were found due to the different halogen substitutions. Finally, it could be observed that all the pyrazolines present an important electrophilic behavior.

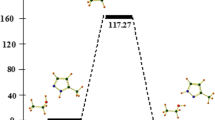
Properties changes in relation to the increasing alkyloxy chain length and halogens presence.












Similar content being viewed by others
References
Wei XQ, Yang G, Cheng JB, Lu ZY, Xie MG (2007) Mater Chem Phys 102(2-3):214. https://doi.org/10.1016/j.matchemphys.2006.12.006
Grabchev I, Chovelon JM (2003) Polym Adv Technol 14(9):601. https://doi.org/10.1002/pat.376
Kumar S, Bawa S, Drabu S, Kumar R, Gupta H (2009) Recent Pat Antiinfect Drug Discov 4 (3):154. https://doi.org/10.2174/157489109789318569
Venkataraman S, Jain S, Shah K, Upmanyu N (2010) Acta Poloniae Pharmaceutica - Drug Research 67(4):361
Karthikeyan MS, Holla BS, Kumari NS (2007) Eur J Med Chem 42(1):30. https://doi.org/10.1016/j.ejmech.2006.07.011
Cuadro AM, Elguero J, Navarro P (1985) Chemical & Pharmaceutical Bulletin 33(6):2535. https://doi.org/10.1248/cpb.33.2535
Bano S, Javed K, Ahmad S, Rathish I, Singh S, Chaitanya M, Arunasree K, Alam M (2013) Eur J Med Chem 65:51. https://doi.org/10.1016/j.ejmech.2013.04.056
Abbas A, Nazir H, Naseer MM, Bolte M, Hussain S, Hafeez N, Hasan A (2014) Spectrochimica Acta Part: A Molecular and Biomolecular Spectroscopy 120:176. https://doi.org/10.1016/j.saa.2013.10.023
Abbas A, Hussain S, Hafeez N, Naseer MM (2014) Spectrochimica Acta Part: A Molecular and Biomolecular Spectroscopy 133:182. https://doi.org/10.1016/j.saa.2014.05.065
Abbas A, Hussain S, Hafeez N, Hasan A, Naseer MM (2014) Spectrochimica Acta Part: A Molecular and Biomolecular Spectroscopy 127:32. https://doi.org/10.1016/j.saa.2014.02.025
Abbas A, Flores-Holguín N, Naseer MM (2015) New J Chem 39(6):4359. https://doi.org/10.1039/c5nj00179j
Chattaraj P, Nath S, Maiti B (2002) In: Bultinck P., De Winter H., Langenaeker W., Tollenaere J.P. (eds) Computational Medicinal Chemistry for Drug Discovery. Marcel Dekker, Inc., New York, pp 295–322
Dennington R, Keith TA, Millam JM (2019) Gaussview Version 5.09. Semichem Inc. Shawnee Mission KS
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr., Peralta JE, Ogliaro F, Bearpark M, Heyd J, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2016) Gaussian 09 Revision E.01 Gaussian Inc., Wallingford CT, 2016
Heyd J, Scuseria GE (2004) J Chem Phys 121(3):1187. https://doi.org/10.1063/1.1760074
Weigend F, Ahlrichs R (2005) Phys Chem Chem Phys 7:3297. https://doi.org/10.1039/b508541a
Weigend F (2006) Phys Chem Chem Phys 8:1057. https://doi.org/10.1039/b515623h
Marenich A, Cramer C, Truhlar D (2009) The Journal of Physical Chemistry B 113:6378. https://doi.org/10.1021/jp810292n
Lewars E (2003) Computational chemistry - Introduction to the theory and applications of molecular and quantum mechanics. Kluwer Academic Publishers, Norwell
Frau J, Glossman-Mitnik D (2017) Frontiers in Chemistry 5:16. https://doi.org/10.3389/fchem.2017.00016
Frau J, Glossman-Mitnik D (2018) Molecules 23(3):559. https://doi.org/10.3390/molecules23030559
Frau J, Glossman-Mitnik D (2018) Theor Chem Acc 137(5):1210. https://doi.org/10.1007/s00214-018-2244-x
Frau J, Glossman-Mitnik D (2018) Frontiers in Chemistry 6(136):1. https://doi.org/10.3389/fchem.2018.00136
Frau J, Glossman-Mitnik D (2018) Contemporary Chemistry 1(1):1
Frau J, Glossman-Mitnik D (2018) Computational and Theoretical Chemistry 1134:22. https://doi.org/10.1016/j.comptc.2018.04.018
Frau J, Glossman-Mitnik D. (2018) Chemical Sciences International Journal 22(4):1. https://doi.org/10.9734/csji/2018/41452
Frau J, Glossman-Mitnik D (2018) J Mol Model 24(138):1. https://doi.org/10.1007/s00894-018-3673-0
Frau J, Flores-Holguín N, Glossman-Mitnik D (2018) Marine Drugs 16(9):302. https://doi.org/10.3390/md16090302
Flores-Holguín N, Frau J, Glossman-Mitnik D (2019) Computational Molecular Bioscience 09(02):41. https://doi.org/10.4236/cmb.2019.92004
Flores-Holguín N, Frau J, Glossman-Mitnik D (2019) In: Stefaniu A. (ed) Cheminformatics and its Applications. IntechOpen, Rijeka, pp 1–8
Parr R, Yang W (1989) Density-functional theory of atoms and molecules. Oxford University Press, New York
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793. https://doi.org/10.1021/cr990029p
Chermette H (1999) J Comput Chem 20:129. https://doi.org/1096-987x(19990115)20:1<129::aid-jcc13>3.0.co;2-a
Gázquez JL, Cedillo A, Vela A (2007) The Journal of Physical Chemistry A 111(10):1966. https://doi.org/10.1021/jp065459f
Chattaraj PK, Sarkar U, Roy DR (2006) Chem Rev 106(6):2065. https://doi.org/10.1021/cr040109f
Domingo L, Ríos-Gutiérrez M, Pérez P (2016) Molecules 21(6):748. https://doi.org/10.3390/molecules21060748
Domingo LR, Aurell M, Pérez P, Contreras R (2002) Tetrahedron 58(22):4417. https://doi.org/10.1016/s0040-4020(02)00410-6
Acknowledgements
This work was supported by Consejo Nacional de Ciencia y Tecnología (CONACYT) and Centro de Investigación de Materiales Avanzados, S.C. (CIMAV). DGM and NFH are researchers from CIMAV and CONACYT.
Funding
AA. gratefully acknowledges the Higher Education Commission (HEC) and Government of Pakistan for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Flores-Holguín, N., Abbas, A. & Glossman-Mitnik, D. Influence on the reactivity properties of the substitution by different halogens on the conjugated backbone of the 1,3,5-triaryl-2-pyrazoline skeleton in relation to the increasing alkyloxy chain length: a conceptual density functional theory study. J Mol Model 26, 174 (2020). https://doi.org/10.1007/s00894-020-04420-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04420-6