Abstract
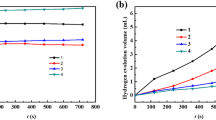
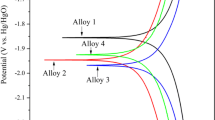
Aluminum (Al) electrodes doped with metals, such as Sn, Ga, and Mg, were prepared by smelting to study the effects of Mg content on the properties of Al electrodes. After testing the corrosion resistance and electrochemical properties (in 4 M NaOH solution) of the Al–0.08Sn–0.08Ga–xMg alloy, the results were compared to virgin Al and an Al–Sn–Ga alloy. The battery performance was studied by constant current discharge. Scanning electron microscope and energy-dispersive X-ray analysis were used to investigate the corrosion form and discharge surface of the alloys. Results showed that the Mg–Sn and Mg–Ga phases act as corrosion centers enabling negative corrosion potential. Electrochemical test results revealed that compared with the pure Al and Al–Sn–Ga alloy, the Al–0.08Sn–0.08Ga–0.5Mg alloy not only showed a more negative potential but also showed enhanced activity. Moreover, it showed high energy density (3169.7 Wh kg−1) and improved anode efficiency (86.9 ± 0.2%) when used as an anode for an aluminum–air battery in NaOH solution.







Similar content being viewed by others
References
Mokhtar M, Talib MZM, Majlan EH et al (2015) Recent developments in materials for aluminum-air batteries: a review. J Ind Eng Chem 32:1–20
Zhan XM, Liu LH, Gao ZN (2011) Electrocatalytic oxidation of quinine sulfate at a multiwall carbon nanotubes-ionic liquid modified glassy carbon electrode and its electrochemical determination. J Solid State Electrochem 15:1185–1192
Zhuk AZ, Sheindlin AE, Kleymenov BV et al (2006) Use of low-cost aluminum in electric energy production. J Power Sources 157:921–926
Kim H, Jeong G, Kim YU et al (2013) Metallic anodes for next-generation secondary batteries. Chem Soc Rev 42:9011–9034
Li Q, Bjerrum NJ (2002) Aluminum as anode for energy storage and conversion: a review. J Power Sources 110:1–10
Yang S, Knickle H (2002) Design and analysis of aluminum/air battery system for electric vehicles. J Power Sources 112:162–173
Kulandainathan MA, Mideen AS, Kapali V et al (1992) Studies on the best alkaline electrolyte for aluminum/air batteries. J Power Sources 39:263–269
Amin MA, El-Rehim SA, El-Sherbini EF et al (2007) The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim Acta 52:3588–3600
Thompson GE, Curioni M, Koroleva EV, et al (2010) Environmentally-friendly anodizing of aluminum alloys. In: Conference of the Australasian Corrosion Association 2010: corrosion and prevention, pp 10–22
Gudić S, Radošević J, Smoljko I et al (2005) Cathodic breakdown of anodic oxide film on Al and Al–Sn alloys in NaCl solution. Electrochim Acta 50:5624–5632
Amin MA, El-Rehim SA, El-Sherbini EF et al (2009) Pitting corrosion studies on Al and Al–Zn alloys in SCN solutions. Electrochim Acta 54:4288–4296
Elango A, Periasamy VM, Paramasivam M (2009) Study on polyaniline-ZnO used as corrosion inhibitors of 57S aluminum in 2 M NaOH solution. Anti-Corros Methods Mater 56:266–270
He J, Wen J, Li X (2011) Effects of precipitates on the electrochemical performance of Al sacrificial anode. Corros Sci 53:1948–1953
Shayeb HAE, Wahab FMAE, Abedin SZE (1999) Role of indium ions on the activation of aluminum. J Appl Electrochem 29:601–609
Doche ML, Novel-Cattin F, Durand R et al (1997) Characterization of different grades of aluminum anodes for aluminum/air batteries. J Power Sources 65:197–205
Sim D, Liu D, Dong X et al (2011) Power factor enhancement for few-layered graphene films by molecular attachments. J Phys Chem C 115:1780–1785
Abedin SZE, Saleh AO (2004) Characterization of some aluminum alloys for application as anodes in alkaline batteries. J Appl Electrochem 34:331–335
Smoljko I (2012) Electrochemical properties of aluminum anodes for Al/air batteries with aqueous sodium chloride electrolyte. J Appl Electrochem 42:969–977
Cabanillas B, Pedrosa M, Cuadrado C et al (2010) Effects of enzymatic hydrolysis on peanut allergenicity. J Allergy Clin Immunol 125:AB224–AB224
Egan DR, León CPD, Wood RJK et al (2013) Developments in electrode materials and electrolytes for aluminum-air batteries. J Power Sources 236:293–310
Wang ZL, Hao XF, Jiang Z et al (2015) C, and N hybrid coordination derived Co–C–N complex as a highly efficient electrocatalyst for hydrogen evolution reaction. J Am Chem Soc 137:15070–15073
Huo K, Gao B, Fu J et al (2014) Fabrication, modification, and biomedical applications of anodized TiO2 nanotube arrays. RSC Adv 4:17300–17324
Fan L, Lu H (2015) The effect of grain size on aluminum anodes for Al–air batteries in alkaline electrolytes. J Power Sources 284:409–415
Srinivas M, Adapaka SK, Neelakantan L (2016) Solubility effects of Sn and Ga on the microstructure and corrosion behavior of Al–Mg–Sn–Ga alloy anodes. J Alloy Compd 683:647–653
Fan L, Lu H, Leng J (2015) Performance of finely structured aluminum anodes in neutral and alkaline electrolytes for Al–air batteries. Electrochim Acta 165:22–28
Sun Z, Lu H, Hong Q et al (2015) Evaluation of an alkaline electrolyte system for Al–air battery. ECS Electrochem Lett 4:A133–A136
Wang J, Li X, Wang Z et al (2013) Enhancement of the electrochemical performance of Al-doped LiVPO4F using AlF3 as aluminum source. J Alloy Compd 581:836–842
Huang Y, Dieringa H, Kainer KU et al (2014) Understanding effects of microstructural inhomogeneity on creep response—new approaches to improve the creep resistance in magnesium alloys. J Magnes Alloys 2:124–132
Wang XM, Hu JM, Zhang JQ et al (2008) Characterization of surface fouling of Ti/IrO electrodes in 4-chlorophenol aqueous solutions by electrochemical impedance spectroscopy. Electrochim Acta 53:3386–3394
Shao HB, Wang JM, Wang XY et al (2004) Anodic dissolution of aluminum in NAOH ethanol solutions. Electrochem Commun 6:6–9
Jun-Guang HE, Wen JB, Xu-Dong LI et al (2011) Influence of Ga and Bi on electrochemical performance of Al–Zn–Sn sacrificial anodes. Trans Nonferr Met Soc China 21:1580–1586
Sherif ESM, Ammar HR, Khalil KA (2014) Effects of copper and titanium on the corrosion behavior of newly fabricated nanocrystalline aluminum in natural seawater. Appl Surf Sci 301:142–148
Osório WR, Spinelli JE, Ferreira IL et al (2007) The roles of macrosegregation and of dendritic array spacing on the electrochemical behavior of an Al–4.5 wt.% Cu alloy. Electrochim Acta 52:3265–3273
Ma J, Wen J, Gao J et al (2014) Performance of Al–1Mg–1Zn–0.1Ga–0.1Sn as anode for Al-air battery. Electrochem Acta 129:69–75
Acknowledgements
The authors are grateful for the financial support from the National Natural Science Foundation of People’s Republic of China (NSFC51761020 and NSFC51761021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare. This research was supported by the National Natural Science Foundation of People’s Republic of China (NSFC51761020 and NSFC51761021). There are no other relationships or activities that could appear to have influenced the submitted work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, S., Tian, C., Alzoabi, S. et al. Performance of an Al–0.08Sn–0.08Ga–xMg alloy as an anode for Al–air batteries in alkaline electrolytes. J Mater Sci 55, 11477–11488 (2020). https://doi.org/10.1007/s10853-020-04711-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04711-6