Abstract
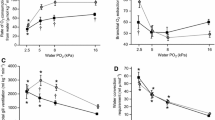
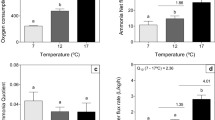
In the pirarucu (Arapaima gigas), gill surface area and thus gas exchange capacity of the gills are reduced with proceeding development. It, therefore, is expected that A. gigas, starting as a water breather, progressively turns into an obligate air-breathing fish using an air-breathing organ (ABO) for gas exchange. We assessed the air-breathing activity, O2 and CO2 exchange into air and water, ammonia-N and urea-N excretion, ion flux rates, and activities of ion transport ATPases in large versus small pirarucu. We found that even very young A. gigas (4–6 g, 2–3 weeks post-hatch) with extensive gills are air-breathers (18.1 breaths*h−1) and cover most (63%) of their O2 requirements from the air whereas 600–700-g animals (about 3–4 months post-hatch), with reduced gills, obtain 75% of their O2 from the air (10.8 breaths*h−1). Accordingly, the reduction in gill surface area hardly affected O2 uptake, but development had a significant effect on aerial CO2 excretion, which was very low (3%) in small fish and increased to 12% in larger fish, yielding a hyper-allometric scaling coefficient (1.12) in contrast to 0.82–0.84 for aquatic and total CO2 excretion. Mass-specific ammonia excretion decreased in approximate proportion to mass-specific O2 consumption as the fish grew, but urea-N excretion dropped from 18% (at 4–6 g) to 8% (at 600–700 g) of total N-excretion; scaling coefficients for all these parameters were 0.70–0.80. Mass-specific sodium influx and efflux rates, as well as potassium net loss rates, departed from this pattern, being greater in larger fish; hyper-allometric scaling coefficients were > 1.0. Gill V-type H+ ATPase activities were greater than Na+, K+-ATPase activities, but levels were generally low and comparable in large and small fish, and similar activities were detected in the ABO. A. gigas is a carnivorous fish throughout its lifecycle, and, despite fasting, protein oxidation accounted for the major portion (61–82%) of aerobic metabolism in both large and small animals. ABO PO2 and PCO2 (measured in 600–700-g fish) were quite variable, and aerial hypoxia resulted in lower ABO PO2 values. Under normoxic conditions, a positive correlation between breath volume and ABP PO2 was detected, and on average with a single breath more than 50% of the ABO volume was exchanged. ABO PCO2 values were in the range of 1.95–3.89 kPa, close to previously recorded blood PCO2 levels. Aerial hypoxia (PO2 down to 12.65 kPa) did not increase either air-breathing frequency or breath volume.







Similar content being viewed by others
References
Bartlett GR (1980) Phosphate compounds in vertebrate red blood cells. Am Zool 20:103–114
Bayley M, Damsgaard C, Thomsen M, Malte H, Wang T (2018) Learning to air-breathe: the first steps. Physiology 34(1):14–29
Boutilier RG, Heming TA, Iwama GK (1984) Appendix: physicochemical parameters for use in respiratory physiology. In: Hoar WS, Randall DJ (eds) Fish physiology. Academic Press, Orlando, pp 403–430
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brauner CJ, Val AL (1996) The interaction between O2 and CO2 exchange in the obligate air-breather Arapaima gigas, and the facultative air breather, Lipossarcus pardalis. In: Val AL, Almeida-Val V, Randall DJ (eds) Physiology and biochemistry of the fishes of the Amazon. INPA, Manaus, pp 101–110
Brauner CJ, Val AL (2006) Oxygen transfer. In: Val AL, Almeida-Val VMF, Randall DJ (eds) The physiology of tropical fishes. Academic Press, Amsterdam, pp 277–306
Brauner CJ, Matey V, Wilson JM, Bernier NJ, Val AL (2004) Transition in organ function during the evolution of air-breathing; insights from Arapaima gigas, an obligate air-breathing teleost from the Amazon. J Exp Biol 207(9):1433–1438
Cameron JN, Wood CM (1978) Renal function and acid-base regulation in two Amazon erythrinid fishes: Hoplias malabaricus, a water breather, and Hoplerythrinus unitaeniatus, a facultative air breather. Can J Zool 56:917–930
Damsgaard C, Baliga VB, Bates E, Burggren W, McKenzie DJ, Taylor E, Wright PA (2019) Evolutionary and cardio‐respiratory physiology of air‐breathing and amphibious fishes. Acta Physiol 228(3):e13406
Dejours P (1981) Principles of comparative respiratory physiology, 2nd edn. Elsevier, Amsterdam
Diaz RJ, Breitburg DL (2009) The hypoxic environment. In: Richards JG, Farrell AP, Brauner CJ (eds) Hypoxia. Elsevier, Amsterdam, pp 1–23
Farrell AP, Randall DJ (1978) Air-breathing mechanics in two Amazonian teleosts, Arapaima gigas and Hoplerythrinus unitaeniatus. Can J Zool 56:939–945
Fernandes MN, da Cruz AL, da Costa OTF, Perry SF (2012) Morphometric partitioning of the respiratory surface area and diffusion capacity of the gills and swim bladder in juvenile Amazonian air-breathing fish, Arapaima gigas. Micron 43(9):961–970
Forgac M (1989) Structure and function of vacuolar class of ATP-driven proton pumps. Physiol Rev 69:765–796
Gonzalez RJ, Brauner CJ, Wang YX, Richards JG, Patrick ML, Xi W, Matey V, Val AL (2010) Impact of ontogenetic changes in branchial morphology on gill function in Arapaima gigas. Physiol Biochem Zool 83(2):322–332
Graham JB (1997) Air-breathing fishes. Evolutions, diversity, and adaptation. Academic Press, London, pp 1–299
Greenwood PH, Liem KF (1984) Aspiratory respiration in Arapaima gigas (Teleostei, Osteoglossomorpha): a reappraisal. J Zool 203(3):411–425
Hochachka PW, Moon TW, Bailey J, Hulbert WC (1978) The osteoglossid kidney: correlations of structure, function, and metabolism with transition to air breathing. Can J Zool 56:820–832
Howell BJ (1970) Acid-base balance in transition from water breathing to air breathing. Fed Proc 29:1130–1134
Hulbert WC, Moon TW, Hochachka PW (1978) The osteoglossid gill: correlations of structure, function, and metabolism with transition to air breathing. Can J Zool 56:801–808
Isaacks RE, Kim HD, Bartlett GR, Harkness DR (1977) Inositol pentaphosphate in erythrocytes of a freshwater fish, piraracu (Arapaima gigas). Life Sci 20(6):987–990
Ishimatsu A (2012) Evolution of the cardiorespiratory system in air-breathing fishes. Aqua-BioSci Monogr 5:1–28
Johansen K, Mangum CP, Weber RE (1978) Reduced blood O2 affinity associated with air breathing in osteoglossid fishes. Can J Zool 56(4):891–897
Kleiber M (1965) Respiratory exchange and metabolic rate. Handbook of physiology. Respiration, vol 3. American Physiological Society, Washington, pp 927–937
Kültz D, Somero GN (1995) Osmotic and thermal effects on in situ ATPase activity in permeabilized gill epithelial cells of the fish Gillichthys mirabilis. J Exp Biol 198:1883–1894
Lauff RF, Wood CH (1996) Respiratory gas exchange, nitrogenous waste excretion, and fuel usage during aerobic swimming in juvenile rainbow trout. J Comp Physiol B 166:501–509
Lee DJ, Gutbrod M, Ferreras FM, Matthews PGD (2018) Changes in hemolymph total CO2 content during the water-to-air respiratory transition of amphibiotic dragonflies. J Exp Biol 221(15):181438
Muusze B, Marcon J, Van den Thillart G, Almeida-Val V (1998) Hypoxia tolerance of Amazon fish respirometry and energy metabolism of the cichlid Astronotus ocellatus. Comp Biochem Physiol Part A 120:151–156
Nawata CM, Hung CCY, Tsui TKN, Wilson JM, Wright PA, Wood CM (2007) Ammonia excretion in rainbow trout (Oncorhynchus mykiss): evidence for Rh glycoprotein and H+-ATPase involvement. Physiol Genomics 31:463–474
Pelster B, Wood CM (2018) Ionoregulatory and oxidative stress issues associated with the evolution of air-breathing. Acta Histochem 120(7):667–679
Pelster B, Wood CM, Speers-Roesch B, Driedzic WR, Almeida-Val V, Val A (2015) Gut transport characteristics in herbivorous and carnivorous serrasalmid fish from ion-poor Rio Negro water. J Comp Physiol B 185(2):225–241
Pelster B, Giacomin M, Wood CM, Val AL (2016) Improved ROS defense in the swimbladder of a facultative air-breathing erythrinid fish, jeju, compared to a non-air-breathing close relative, traira. J Comp Physiol B 186(5):615–624
Pelster B, Wood CM, Jung E, Val AL (2018) Air-breathing behavior, oxygen concentrations, and ROS defense in the swimbladders of two erythrinid fish, the facultative air-breathing jeju, and the non-air-breathing traira during normoxia, hypoxia and hyperoxia. J Comp Physiol B 188(3):437–449
Perry SF, Gilmour KM, Swenson ER, Vulesevic B, Chew SF, Ip YK (2005) An investigation of the role of carbonic anhydrase in aquatic and aerial gas transfer in the African lungfish Protopterus dolloi. J Exp Biol 208(19):3805–3815
Perry SF, Jonz MG, Gilmour KM (2009) Oxygen sensing and the hypoxic ventilatory response. In: Richards JG, Farrell AP, Brauner CJ (eds) Hypoxia. Academic Press, Amsterdam, pp 193–253
Rahmatullah M, Boyde TR (1980) Improvements in the determination of urea using diacetyl monoxime; methods with and without deproteinisation. Clin Chim Acta 107:3–9
Rahn H (1966) Aquatic gas exchange: theory. Respir Physiol 1(1):1–12
Ramos CA, Fernandes MN, da Costa OTF, Duncan WP (2013) Implications for osmorespiratory compromise by anatomical remodeling in the gills of Arapaima gigas. Anat Rec 296(10):1664–1675
Randall DJ, Farrell AP, Haswell MS (1978) Carbon dioxide excretion in the pirarucu (Arapaima gigas), an obligate air-breathing fish. Can J Zool 56:977–982
Randall DJ, Cameron JN, Daxboeck C, Smatresk N (1981) Aspects of bimodal gas exchange in the bowfin, Amia calva L. (Actinopterygii: Amiiformes). Respir Physiol 43(3):339–348
Scott GR, Matey V, Mendoza JA, Gilmour KM, Perry SF, Almeida-Val VMF, Val AL (2017) Air breathing and aquatic gas exchange during hypoxia in armoured catfish. J Comp Physiol B 187(1):117–133
Shartau RB, Brauner CJ (2014) Acid–base and ion balance in fishes with bimodal respiration. J Fish Biol 84(3):682–704
Smatresk NJ (1986) Ventilatory and cardiac reflex responses to hypoxia and NaCN in Lepisosteus osseus, an air-breathing fish. Physiol Zool 59(4):385–397
Smatresk NJ, Cameron JN (1982) Respiration and acid-base physiology of the spotted gar, a bimodal breather: I. Normal values, and the response to severe hypoxia. J Exp Biol 96(1):263–280
Stevens ED, Holeton GF (1978) The partitioning of oxygen uptake from air and from water by the large obligate air-breathing teleost pirarucu (Arapaima gigas). Can J Zool 56(4):974–976
Val AL, Almeida-Val VMF (1995) Fishes of the Amazon and their environment. Springer-Verlag, Berlin
Val AL, Affonso EG, Souza RHS, Almeida-Val VMF, Moura MAF (1992) Inositol pentaphosphate in the erythrocytes of and Amazonian fish, the pirarucu (Arapaima gigas). Can J Zool 70:852–855
Verdouw H, van Echted CJA, Dekkers EMJ (1978) Ammonia determination based on indophenol formation with sodium salicylate. Water Res 12:399–402
Welker AF, Moreira DC, Campos EG, Hermes-Lima M (2013) Role of redox metabolism for adaptation of aquatic animals to drastic changes in oxygen availability. Comp Biochem Physiol A Mol Integr Physiol 165(4):384–404
Wood CM (1992) Flux measurements as indices of H+ and metal effects on freshwater fish. Aquat Toxicol 22:239–264
Wood CM (1996) Is there a relationship between urea production and acid–base balance in fish? In: Val AL, Randall DJ (eds) Physiology and biochemistry of the fishes of the Amazon. INPA, Manaus, pp 339–357
Wood CM (2001) The influence of feeding, exercise, and temperature on nitrogen metabolism and excretion. In: Anderson PA, Wright PA (eds) Fish physiology, vol 20. Academic Press, Orlando, pp 201–238
Wood CM, Eom J (2019) The internal CO2 threat to fish: high PCO2 in the digestive tract. Proc Biol Sci 286(1907):20190832
Wood CM, Pelster B, Giacomin M, Sadauskas-Henrique H, Almeida-Val VM, Val AL (2016) The transition from water-breathing to air-breathing is associated with a shift in ion uptake from gills to gut: a study of two closely related erythrinid teleosts, Hoplerythrinus unitaeniatus and Hoplias malabaricus. J Comp Physiol B 186(4):431–445
Wright PA, Fyhn HJ (2001) Ontogeny of nitrogen metabolism and excretion. Fish physiology nitrogen excretion, 20th edn. Academic Press, San Diego, pp 149–200
Zall DM, Fisher D, Garner MQ (1956) Photometric determination of chloride in water. Anal Chem 28:1665–1668
Zimmer AM, Wright PA, Wood CM (2017) Ammonia and urea handling by early life stages of fish: a review. J Exp Biol 220:3843–3855
Acknowledgements
Financial support by INCT ADAPTA-CNPq (465540/2014-7)/FAPEAM (062.1187/2017)/CAPES (finance code 001), Science without Borders (Brazil), and NSERC Discovery (Canada) (RGPIN-2017-03843) is gratefully acknowledged. ALV is the recipient of a research fellowship from Brazilian CNPq. We thank Drs. Raul Suarez and John Onukwufor for advice on allometry calculations. This research was made possible by the generous gift of prototype PCO2 micro-fibre-optodes, opto-electronic meter, and software by PreSens Precision Sensing GmbH. We thank Fernando Martinez Ferreras, Miriam Ubach Granados, and Laura Perez Medina of PreSens Precision Sensing GmbH for advice and support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pelster, B., Wood, C.M., Braz-Mota, S. et al. Gills and air-breathing organ in O2 uptake, CO2 excretion, N-waste excretion, and ionoregulation in small and large pirarucu (Arapaima gigas). J Comp Physiol B 190, 569–583 (2020). https://doi.org/10.1007/s00360-020-01286-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-020-01286-1