Abstract
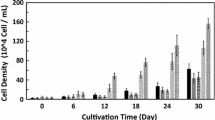
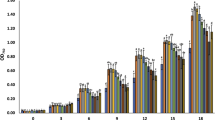
In microalga culturing, one important topic is finding methods of increasing the productivity of algal biomass grown on a large scale. The cultured microalgal biomass must be inexpensive to produce, but rich in the bioactive substances, for use as raw materials for many different purposes, such as functional foods, drugs, pharmaceuticals, cosmetics, and biofuels. Here we present results about the effects of ferulic acid extracted from rice bran on the growth, photosynthetic activity, and valuable substance content of the eustigmatophyte Nannochloropsis oculata. The results showed that ferulic acid is a growth promoter that increases N. oculata biomass. Addition of 100 mg L−1 ferulic acid to the culture medium led to an increase in cell density and specific growth rate (μ; day−1) of N. oculata. These values were 2.52 and 2.02 times higher, respectively, than those in the control group (without the addition of ferulic acid). Moreover, photosynthetic parameters, lipid and carbohydrate contents, and intracellular pigment contents (such as chlorophyll a and carotenoids) in the treated algal biomass tended to be higher than those in the control treatment. This study provides an initial scientific basis for the improvement of algal biomass productivity and metabolite production in safe and sustainable ways with naturally derived substances.



Similar content being viewed by others
References
Arora S, Mishra G (2019) Biochemical modulation of Monodopsis subterranea (Eustigmatophyceae) by auxin and cytokinin enhances eicosapentaenoic acid productivity. J Appl Phycol 31:3441–3452
Blackburn SI, Volkman JK (2012) Microalgae: a renewable source of bioproducts. In: Dunford NT (ed) Food and industrial bioproducts and bioprocessing. Wiley-Blackwell, Iowa, pp 221–241
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Phys 37:911–917
Borowitzka MA, Borowitzka LJ, Kessly D (1990) Effects of salinity increase on carotenoid accumulation in the green alga Dunaliella salina. J Appl Phycol 2:111–119
Bradford MM (1976) Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cho K, Kim KN, Lim NL, Kim MS, Ha JC, Shin HH, Kim MK, Roh SW, Kim D, Oda T (2015) Enhanced biomass and lipid production by supplement of myo-inositol with oceanic microalga Dunaliella salina. Biomass Bioenergy 72:1–7
Cooke MS (2003) Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J 17:1195–1214
Cosgrove J, Borowitzka MA (2011) Chlorophyll fluorescence terminology: an introduction. In: Suggett DJ, Prásil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications. Springer, Dordrecht, pp 1–17
Dang DH, Hoang MH, Nguyen DH, Hoang SN, Hoang LA, Ngo HT, Dinh KC (2007) Research on biosynthesis of DHA from new heterotrophic marine algae Labyrinthula, Schizochytrium and its application. J Sci Technol 45:144–154 (in Vietnamese)
Dang DH, Hoang TLA, Luu TT, Pau LS, Hui YL (2019) Effect of nanoscale zerovalent cobalt on growth and photosynthetic parameters of soybean Glycine max (L.) Merr. DT26 at different stages. BMC Energy 1:6
De Klerk GJ, Guan H, Huisman P, Marinova S (2011) Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul 63:175–185
Dos Santos WD, Ferrarese MLL, Ferrarese-Filho O (2008) Ferulic acid: an allelochemical troublemaker. Funct Plant Sci Biotechnol 2:47–55
Ferrarese MLL, Souza NE, Rodrigues JD, Ferrarese-Filho O (2001) Carbohydrate and lipid status in soybean roots influenced by ferulic acid uptake. Acta Physiol Plantarum 23:421–427
Hashtroudi MS, Ghassempour A, Riahi H, Shariatmadari Z, Khanjir M (2012) Endogenous auxins in plant growth-promoting Cyanobacteria - Anabaena vaginicola and Nostoc calcicola. J Appl Phycol 25:379–386
Imamoglu E, Sukan FV, Dalay MC (2007) Effect on different culture media and light intensities on growth of Haematococcus pluvialis. Int J Nat Eng Sci 1:05–09
Kilic NK, Erdem K, Donmez G (2018) Bioactive compounds produced by Dunaliella species, antimicrobial effects and optimization of the efficiency. Turk J Fish Aquat Sci 19:923–933
Kitajima M, Butler WL (1975) Quenching of chlorophyll fluorescence and primary photochemistry in chloroplasts by dibromothymoquinone. Biochim Biophys Acta 376:105–115
Kozlova TA, Hardy BP, Krishna P, Levin DB (2017) Effect of phytohormones on growth and accumulation of pigments and fatty acids in the microalgae Scenedesmus quadricauda. Algal Res 27:325–334
Li Y, Lian S, Tong D, Song R, Yang W, Fan Y, Qing R, Hu C (2011) One-step production of biodiesel from Nannochloropsis sp. on solid base Mg-Zr catalyst. Appl Energy 88:3313–3317
Lichtenthaler HK (1994) Chlorophylls and carotenoids pigments of photosynthetic. Methods Enzymol 148:350–382
Liu XF, Hu XJ (2001) Effects of allelochemical ferulic acid on endogenous hormone level of wheat seedling. Chin J Ecol Agric 9:96–98
Mallick N, Mohn FH (2000) Reactive oxygen species: response of algal cells. J Plant Physiol 157:183–193
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mohan K, Phale PS (2017) Carbon source-dependent inducible metabolism of veratryl alcohol and ferulic acid in Pseudomonas putida CSV86. Appl Environ Microbiol 83:e03326–e03316
Nakai S (2001) Algal growth inhibition effects and inducement modes by plant-producing phenols. Water Res 35:1855–1859
Nogami R, Nishida H, Hong DD, Wakisaka M (2016) Growth promotion of Spirulina by steelmaking slag: application of solubility diagram to understand its mechanism. AMB Express 6:96
Nogami R, Nishida H, Hong DD, Wakisaka M (2017) Growth promotion effect of alginate oligosaccharides to Spirulina by repeated batch culture. J Jap Inst Energy 96:352–356
Park MH, Han MS, Ahn CY, Kim HS, Yoon BD, Oh HM (2006) Growth inhibition of bloom forming cyanobacterium Mycrocystis aeruginosa by rice straw extract. Lett Appl Microbiol 43:307–312
Qiu Z, Wang L, Zhou Q (2013) Effects of bisphenol A on growth, photosynthesis and chlorophyll fluorescence in above-ground organs of soybean seedlings. Chemosphere 90:1274–1280
Roy S, Metya SK, Sannigrahi S, Rahaman N, Ahmed F (2013) Treatment with ferulic acid to rats with streptozotocin-induced diabetes: effects on oxidative stress, pro-inflammatory cytokines, and apoptosis in the pancreatic β cell. Endocrine 44:369–379
Singh HP, Kaur S, Batish DR, Kohli RK (2014) Ferulic acid impairs rhizogenesis and root growth, and alters associated biochemical changes in mung bean (Vigna radiata) hypocotyls. J Plant Interact 9:267–274
Sistiafi A, Putri D (2018) Biodiesel synthesis from Nannochloropsis oculata and Chlorella vulgaris through transesterification process using NaOH/zeolite heterogeneous catalyst. IOP Conf. Ser.: Earth Environ Sci
Srinivas R, Ochs CA (2012) Effect of UV-A irradiance on lipid accumulation in Nannochloropsis oculata. Photochem Photobiol 88:684–689
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Yang GW, Jiang JS, Lu WQ (2015) Ferulic acid exerts anti-angiogenic and antitumor activity by targeting fibroblast growth factor receptor 1-mediated angiogenesis. Int J Mol Sci 16:24011–24031
Zduńska K, Dana A, Kolodziejczak A, Rotsztejn H (2018) Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol Physiol 31:332–336
Zeng RS, Luo SM, Shi YH, Shi MB, Tu CY (2001) Physiological and biochemical mechanism of allelopathy of secalonic acid F on higher plants. Agron J 93:72–79
Zhang X, Yuan H, Guan L, Wang X, Wang Y, Jiang Z, Cao L, Zhang X (2019) Influence of photoperiods on microalgae biofilm: photosynthetic performance, biomass yield, and cellular composition. Energies 12:3724
Zhu J, Wakisaka M (2018) Growth promotion of Euglena gracilis by ferulic acid from rice bran. AMB Express 8:16
Acknowledgments
The authors would like to thank Prof. Dr. Choon-Hwan Lee (Pusan National University, Korea) for the use of his Mini-PAM II chlorophyll fluorometer and for his valuable information about using the PAM on microalgae.
Funding
This work was supported by a project under the program of supporting senior researchers with Code NVCC08.14/19-19 (for Prof. Dr. Dang Diem Hong).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tam, L.T., Ha, N.C., Thom, L.T. et al. Ferulic acid extracted from rice bran as a growth promoter for the microalga Nannochloropsis oculata. J Appl Phycol 33, 37–45 (2021). https://doi.org/10.1007/s10811-020-02166-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02166-5