Abstract
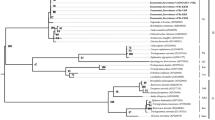
Tetranychidae spider mites are considered key citrus pests in some production areas, especially Tetranychus urticae Koch. Over the past decades, pesticide overuse seems to have promoted T. urticae population selection in citrus orchards. However, the microbiota has also been pointed out as a plausible explanation for population structure or plant host specialisation observed in several arthropod species. In this work, we have determined the incidence of Cardinium, Rickettsia, Spiroplasma and Wolbachia as representatives of major distorter bacteria genera in Aplonobia histricina (Berlese), Eutetranychus banksi (McGregor), Eutetranychus orientalis (Klein), Panonychus citri (McGregor), Tetranychus evansi Baker and Pritchard, Tetranychus turkestani Ugarov and Nikolskii, and T. urticae populations from Spanish citrus orchards. Only Wolbachia was detected by PCR. The multilocus alignment approach and phylogenetic inference indicated that all detected Wolbachia belong to supergroup B. The deep analysis of each 16S rDNA, ftsZ and wsp gene sequences allowed identifying several phylogenetically different Wolbachia sequences. It probably indicates the presence of several different races or strains, all of them belonging to supergroup B. The wsp sequence typing analysis unveiled the presence of the two already identified alleles (61 and 370) and allowed to contribute with five new alleles, supporting the presence of different but related B-races in the studied mite populations. The results are discussed and related to T. urticae population structure, previously observed in Spanish citrus orchards.






Similar content being viewed by others
References
Aguilar-Fenollosa E, Pina T, Gómez-Martínez MA, Hurtado MA, Jacas JA (2012) Does host adaptation of Tetranychus urticae populations in clementine orchards with a Festuca arundinacea cover contribute to a better natural regulation of this pest mite? Entomol Exp Appl 144(2):181–190
Aguilar-Fenollosa E, Rey-Caballero J, Blasco JM, Segarra-Moragues JG, Hurtado MA, Jacas JA (2016) Patterns of ambulatory dispersal in Tetranychus urticae can be associated with host plant specialization. Exp Appl Acarol 68:1–20
Ahmed MZ, Li S-L, Xue X, Yin Y-J, Ren S-X, Jiggins FM, Greeff JM, Qiu B-L (2015) The intracellular bacterium Wolbachia uses parasitoid wasps as phoretic vectors for efficient horizontal transmission. PLoS Pathog 10(2):e1004672–e1004672. https://doi.org/10.1371/journal.ppat.1004672
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25(17):3389–3402
Atyame CM, Pasteur N, Dumas E, Tortosa P, Tantley ML, Pocquet N, Licciardi S, Bheecarry A, Zumbo B, Weill M, Duron O (2011) Cytoplasmic incompatibility as a means of controlling Culex pipiens quinquefasciatus mosquito in the islands of the South-Western Indian ocean. PLoS Negl Trop Dis 5(12):e1440. https://doi.org/10.1371/journal.pntd.0001440
Augustinos AA, Santos-Garcia D, Dionyssopoulou E, Moreira M, Papapanagiotou A, Scarvelakis M, Doudoumis V, Ramos S, Aguiar AF, Borges PAV, Khadem M, Latorre A, Tsiamis G, Bourtzis K (2011) Detection and characterization of Wolbachia infections in natural populations of aphids: is the hidden diversity fully unraveled? PloS ONE 6(12):e28695. https://doi.org/10.1371/journal.pone.0028695
Baldo L, Lo N, Werren JH (2005) Mosaic nature of the Wolbachia surface protein. J Bacteriol 187(5):5406–5418. doi:https://doi.org/10.1128/JB.187.15.5406-5418.2005
Baldo L, Hotopp JCD, Jolley KA, Bordenstein SR, Biber SA, Choudhoury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH (2006) Multilocus sequence typing systems for endosymbiont Wolbachia pipientis. Appl Environ Microbiol 72:7098–7110
Baldo L, Prendini L, Corthals A, Werren JH (2007) Wolbachia are present in Southern African scorpions and cluster with supergroup F. Curr Microbiol 55:367–373
Bleidorn C, Gerth M (2018) A critical re-evaluation of multilocus sequence typing (MLST) efforts in Wolbachia. FEMS Microbiol Ecol 94(1):fix163. https://doi.org/10.1093/femsec/fix163
Bolanos LM, Servin-Garciduenas LE, Martinez-Romero E (2015) Arthropod–Spiroplasma relationship in the genomic era. FEMS Microbiol Ecol 91(2):1–8
Bordenstein S, Rosengaus RB (2005) Discovery of a novel Wolbachia supergroup in Isoptera. Curr Microbiol 51:393–398
Boubou A, Migeon A, Roderick GK, Navajas M (2011) Recent emergence and worldwide spread of the red tomato spider mite, Tetranychus evansi: genetic variation and multiple cryptic invasions. Biol Invas 13(1):81–92. https://doi.org/10.1007/s10530-010-9791-y
Boubou A, Migeon A, Roderick GK, Auger P, Cornuet JM, Magalhães S, Navajas M (2012) Test of colonisation scenarios reveals complex invasion history of the red tomato spider mite Tetranychus evansi. PLoS ONE 7(4):e35601. https://doi.org/10.1371/journal.pone.0035601
Braig HR, Zhou W, Dobson SL, O’Neill SL (1998) Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J Bacteriol 180(9):2373–2378
Brelsfoard CL, Dobson SL (2009) Wolbachia-based strategies to control insect pests and disease vectors. Asia Pac J Mol Biol Biotechnol 17(3):55–63
Buchner P (1965) Endosymbiosis of animals with plant microorganisms. Wiley, New York
Casiraghi M, Bordenstein SR, Baldo L, Lo N, Beninati T et al (2005) Phylogeny of Wolbachia pipientis based on gltA, groEL and ftsZ gene sequences: clustering of arthropod and nematode symbionts in the F supergroup, and evidence for further diversity in the Wolbachia tree. Microbiol 151:4015–4022
Chu C-C, Hoffmann M, Braswell WE, Pelz-Stelinski KS (2019) Genetic variation and potential coinfection of Wolbachia among widespread Asian citrus psyllid (Diaphorina citri Kuwayama) populations. Insect Sci 26:671–682
Duron O, Bouchon D, Boutin S, Bellamy L, Zhou L, Engelstädter J, Hurst GD (2008) The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biol 6:27
Duron O, Hurst GD (2013) Arthropods and inherited bacteria: from counting the symbionts to understanding how symbionts count. BMC Biol 11(1):45
Engelstadter J, Hurst GDD (2009) The ecology and evolution of microbes that manipulate host reproduction. Ann Rev Ecol Evol Syst 40:127–149
Enigl M, Schausberger P (2007) Incidence of the endosymbionts Wolbachia, Cardinium and Spiroplasma in phytoseiid mites and associated prey. Exp Appl Acarol 42(2):75–85
Frago E, Mala M, Weldegergis BT, Yang C, McLean A, Godfray HCJ, Gols R, Dicke M (2017) Symbionts protect aphids from parasitic wasps by attenuating herbivore-induced plant volatiles. Nat Commun 8(1):1860
Gotoh T, Noda H, Hong XY (2003) Wolbachia distribution and cytoplasmic incompatibility based on a survey of 42 spider mite species (Acari: Tetranychidae) in Japan. Heredity 91:208–216
Gotoh T, Noda H, Ito S (2007a) Cardinium symbionts cause cytoplasmic incompatibility in spider mites. Heredity 98(1):13–20
Gotoh T, Sugasawa J, Noda H, Kitashima Y (2007b) Wolbachia-induced cytoplasmic incompatibility in Japanese populations of Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 42:1–16
Gottlieb Y, Ghanim M, Chiel E, Gerling D, Portnoy V, Steinberg S, Tzuri G, Horowitz AR, Belausov E, Mozes-Daube N, Kontsedalov S, Gershon M, Gal S, Katzir N, Zchori-Fein E (2006) Identification and localization of a Rickettsia sp. in Bemisia tabaci (Homoptera: Aleyrodidae). Appl Environ Microbiol 72(5):3646–3652
Guidolin AS, Cataldi TR, Labate CA, Francis F, Cônsoli FL (2018) Spiroplasma affects host aphid proteomics feeding on two nutritional resources. Sci Rep 8(1):2466
Heyworth ER, Ferrari J (2015) A facultative endosymbiont in aphids can provide diverse ecological benefits. J Evol Biol Res 28(10):1753–1760
Hosokawa T, Koga R, Kikuchi Y, Meng XY, Fukatsu T (2010) Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc Natl Acad Sci USA 107:769–774
Jacas JA, Karamaouna F, Vercher R, Zappalà L (2010) Citrus pest management in the Northern Mediterranean basin: Spain, Italy and Greece. In: Ciancia A, Mukerji KG (eds) Integrated management of arthropod pests and insect borne diseases, vol 5. Springer, The Netherlands, pp 3–26
Jaques JA, Aguilar-Fenollosa E, Hurtado-Ruiz MA, Pina T (2015) Food web engineering to enhance biological control of tetranychus urticae by phytoseiid mites (Tetranychidae: Phytoseiidae) in Citrus. In: Carrillo D, de Moraes G, Peña J (eds) Prospects for biological control of plant feeding mites and other harmful organisms. Progress in biological control. Springer, Cham, pp 251–269
Jeyaprakash A, Hoy MA (2000) Long PCR improves Wolbachia DNA amplifications: wsp sequences found in 76% of sixty-three arthropod species. Insect Mol Biol 9:393–405
Jolley KA, Maiden MCJ (2010) BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinf 11:595
Kliot A, Cilia M, Czosnek H, Ghanim M (2014) Implication of the bacterial endosymbiont Rickettsia spp. in interactions of the whitefly Bemisia tabaci with tomato yellow leaf curl virus. J Virol 88(10):5652–5660
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Li S-J, Ahmed MZ, Lv N, Shi P-Q, Wang X-M, Huang J-L, Qiu B-L (2017) Plant-mediated horizontal transmission of Wolbachia between whiteflies. ISME J 11:1019–1028
Liu X-D, Guo H-F (2019) Importance of endosymbionts Wolbachia and Rickettsia in insect resistance development. Curr Opin Insect Sci 33:84–90
Lo N, Casiraghi M, Salati E, Bazzocchi C, Bandi C (2002) How many Wolbachia supergroups exist? Mol Biol Evol 19(3):341–346
Lo N, Paraskevopoulos C, Bourtzis K, O’Neill SL, Werren JH, Bordenstein SR, Bandi C (2007) Taxonomic status of the intracellular bacterium Wolbachia pipientis. Int J Syst Evol Micro 57(3):654–657
Marinosci C, Magalhaes S, Macke E, Navajas M, Carbonell D, Devaux C, Olivieri I (2015) Effects of host plant on life-history traits in the polyphagous spider mite Tetranychus urticae. Ecol Evol 5(15):3151–3158
Mathé-Hubert H, Kaech H, Ganesanandamoorthy P, Vorburger C (2019) Evolutionary costs and benefits of infection with diverse strains of Spiroplasma in pea aphids. Evolution 73(7):1466–1481
Migeon A, Dorkeld F (2019) Spider Mites Web: a comprehensive database for the Tetranychidae. http://www1.montpellier.inra.fr/CBGP/spmweb/. Accessed 20 Oct 2019
Montenegro H, Souza WN, Leite DS, Klaczko LB (2000) Male-killing selfish cytoplasmic element causes sex-ratio distortion in Drosophila melanogaster. Heredity 85:465–470
Monzó C, Sabater-Muñoz B, Urbaneja A, Castañera P (2010) Tracking medfly predation by the wolf spider, Pardosa cribata Simon, in citrus orchards using PCR-based gut-content analysis. Bull Entomol Res 100(2):145–152
Nicholas KB, Nicholas HBJ (1994–1998) GENEDOC: a tool for editing and annotation multiple sequence alignments. www.psc.edu/biomed/genedoc
Osaka R, Ichizono T, Kageyama D, Nomura M, Watada M (2013) Natural variation in population densities and vertical transmission rates of a Spiroplasma endosymbiont in Drosophila hydei. Symbiosis 60:73–78
Oliver KM, Degnan PH, Burke GR, Moran NA (2010) Facultative symbionts in aphids and the horizontal transfer of ecologically important traits. Annu Rev Entomol 55:247–266
O’Neill S, Giordano R, Colbert AME, Karr TL, Robertson HM (1992) 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc Natl Acad Sci USA 89:2699–2702
Pascar J, Chandler CH (2018) A bioinformatics approach to identifying Wolbachia infections in arthropods. PeerJ 6:e5486. https://doi.org/10.7717/peerj.5486
Pascual-Ruiz S, Gómez-Martinez MA, Ansaloni T, Segarra-Moragues JG, Sabater-Muñoz B, Jacas JA, Hurtado-Ruiz MA (2014) Genetic structure of a phytophagous mite species affected by crop practices: the case of Tetranychus urticae in clementine mandarins. Exp Appl Acarol 62:477–498
Pérez-Sayas C, Pina T, Gómez-Martínez MA, Camañes G, Ibáñez-Gual MV, Jaques JA, Hurtado MA (2015) Disentangling field mite predator-prey relationships by multiplex PCR. Mol Ecol Res 15(6):1330–1345
Perlman SJ, Hunter MS, Zchori-Fein E (2006) The emerging diversity of Rickettsia. Proc R Soc Lond B Biol Sci 273(1598):2097–2106
Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA (2018) Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Syst Biol 6785:901–904. https://doi.org/10.1093/sysbio/syy032
Ros VID, Fleming VM, Feil EJ, Breeuwer JAJ (2009) How diverse is the genus Wolbachia? Multiple-gene sequencing reveal a putatively new Wolbachia supergroup recovered from spider mites (Acari: Tetranychidae). Appl Environ Microbiol 75:1036–1043
Ros VID, Fleming VM, Feil EJ, Breeuwer JAJ (2012) Diversity and recombination in Wolbachia and Cardinium from Bryobia spider mites. BMC Microbiol 12(S1):S13. https://doi.org/10.1186/1471-2180-12-S1-S13
Russell JA, Latorre A, Sabater-Munoz B, Moya A, Moran NA (2003) Side-stepping secondary symbionts: widespread horizontal transfer across and beyond the Aphidoidea. Mol Ecol 12(4):1061–1075
Sato Y, Staudacher H, Sabelis MW (2016) Why do males choose heterospecific females in the red spider mite? Exp Appl Acarol 68(1):21–31
Schulenburg JGvd, Hurst GDD, Huigens TME, van Meer MMM, Jiggings FM, Majerus MEN (2000) Molecular evolution and phylogenetic utility of Wolbachia ftsZ and wsp gene sequences with special reference to the origin of male-killing. Mol Biol Evol 14(4):584–600
Sintupachee S, Milne JR, Poonchaisri S, Baimai V, Kittayapong P (2006) Closely related Wolbachia strains within the pumpkin arthropod community and the potential for horizontal transmission via the plant. Microb Ecol 51(3):294–301
Staden R (1996) The Staden sequence analysis package. Mol Biotechnol 5(3):233
Staudacher H, Schimmel BCJ, Lamers MM, Wybouw N, Groot AT, Kant MR (2017) Independent effects of a herbivore’s bacterial symbionts on its performance and induced plant defences. Int J Mol Sci 18:182. https://doi.org/10.3390/ijms18010182
Stevens L, Giordano R, Fialho RF (2001) Male-killing, nematode infections, bacteriophage infection, and virulence of cytoplasmic bacteria in the genus Wolbachia. Annu Rev Ecol Syst 32:519–545
Stouthamer R, Breeuwer JA, Hurst GD (1999) Wolbachia pipientis: microbial manipulator of arthropod reproduction. Annu Rev Microbiol 53:71–102
Suchard MA, Lemey P, Baele G, Ayres DL, Drummond AJ, Rambaut A (2018) Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol 4:vey016
Suh E, Sim C, Park JJ, Cho K (2015) Inter-population variation for Wolbachia induced reproductive incompatibility in the haplodiploid mite Tetranychus urticae. Exp Appl Acarol 65:55–71
van Ham RC, Moya A, Latorre A (1997) Putative evolutionary origin of plasmids carrying the genes involved in leucine biosynthesis in Buchnera aphidicola (endosymbiont of aphids). J Bacteriol 179(15):4768–4777
van Meer MMM, Witteveldt J, Stouthamer R (1999) Phylogeny of the arthropod endosymbiont Wolbachia based on the wsp gene. Insect Mol Biol 8(3):399–408
Weinert LA, Werren JH, Aebi A, Stone GN, Jiggins FM (2009) Evolution and diversity of Rickettsia bacteria. BMC Biol 7:6. https://doi.org/10.1186/1741-7007-7-6
Weinert LA, Araujo-Jnr EV, Ahmed MZ, Welch JJ (2015) The incidence of bacterial endosymbionts in terrestrial arthropods. Proc R Soc Lond B Biol Sci 282(1807):20150249. https://doi.org/10.1098/rspb.2015.0249
Werren JH (1997) Biology of Wolbachia. Annu Rev Entomol 42(1):587–609
Xie RR, Chen XL, Hong XY (2011) Variable fitness and reproductive effects of Wolbachia infection in populations of the two-spotted spider mite Tetranychus urticae Koch in China. Appl Entomol Zool 46:95–102
Xie RR, Sun JT, Xue XF, Hong XY (2016) Cytoplasmic incompatibility and fitness benefits in the two-spotted spider mite Tetranychus urticae (red form) doubly infected with Wolbachia and Cardinium. Syst Appl Acarol 21(9):1161–1174
Zabalou S, Riegler M, Theodorakopoulou M, Stauffer C, Savakis C (2004) Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control. Proc Natl Acad Sci USA 101(42):15042–15045
Zchori-Fein E, Perlman SJ (2004) Distribution of the bacterial symbiont Cardinium in arthropods. Mol Ecol 13(7):2009–2016
Zchori-Fein E, Perlman SJ, Kelly SE, Katzir N, Hunter MS (2004) Characterization of a Bacteroidetes symbiont in Encarsia wasps (Hymenoptera: Aphelinidae): proposal of ‘Candidatus cardinium hertigii’. Int J Syst Evol Microbiol 54:961–968. https://doi.org/10.1099/ijs.0.02957-0
Zélé F, Weill M, Magalhães S (2018a) Identification of spider mite species and their endosymbionts using multiplex PCR. Exp Appl Acarol 74(2):123–138
Zélé F, Santos I, Olivier I, Weill M, Duron O, Magalhães S (2018b) Endosymbiont diversity and prevalence in herbivorous spider mite populations in South-Western Europe. FEMS Microbiol Ecol 94(4):fiy015. https://doi.org/10.1093/femsec/fiy015
Zhang YK, Zhang KJ, Sun JT, Yang XM, Ge C, Hong XY (2013) Diversity of Wolbachia in natural populations of spider mites (genus Tetranychus): evidence for complex infection history and disequilibrium distribution. Microb Ecol 65(3):731–739
Zhang YK, Chen YT, Yang K, Qiao GX, Hong XY (2016) Screening of spider mites (Acari: Tetranychidae) for reproductive endosymbionts reveals links between co-infection and evolutionary history. Sci Rep 6:27900. https://doi.org/10.1038/srep27900
Zhao DX, Zhang XF, Hong XY (2013) Host-symbiont interactions in spider mite Tetranychus truncatus doubly infected with Wolbachia and Cardinium. Environ Entomol 42(3):445–452
Zhou W, Rousset F, O’Neill S (1998) Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc R Soc Lond B 265:509–515
Zhou X-F, Li Z-X (2016) Establishment of the cytoplasmic incompatibility-inducing Wolbachia strain wMel in an important agricultural pest insect. Sci Rep 6:39200. https://doi.org/10.1038/srep39200
Zhu LY, Zhang KJ, Zhang YK, Ge C, Gotoh T, Hong XY (2012) Wolbachia strengthens Cardinium-induced cytoplasmic incompatibility in the spider mite Tetranychus piercei McGregor. Curr Microbiol 65:516–523
Zhu YX, Song YL, Zhang YK, Hoffmann AA, Zhou JC, Sun JT, Hong XY (2018) Incidence of facultative bacterial endosymbionts in spider mites associated with local environments and host plants. Appl Environ Microbiol 84(6):e02546–e02517
Zug R, Hammerstein P (2012) Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE 7:e38544. https://doi.org/10.1371/journal.pone.0038544
Acknowledgements
We would like to acknowledge F.J. Beitia (Valencian Institute of Agricultural Research (IVIA), Spain), C. García and R. Gil (University of Valencia (UV), Spain), S. Perlman and M. Ballinger (University of Victoria, Canada), and D. Santos-Garcia, S. Morin and Y. Gottlieb-Dror (The Hebrew University of Jerusalem, Israel) for providing insects with known infections by Cardinium, Rickettsia, Spiroplasma or Wolbachia, used as positive controls. Polyphagotarsonemus latus specimens were obtained from D. Peris (University Jaume I (UJI), Spain) rearing colony. We would like to acknowledge the help of the undergraduate students P. Ruiz Guillem and P. Bonilla Villamil (UV), E. Pallarés Gual and M. López-Martínez (UJI), in DNA extractions and PCRs, and to B. Hurtado for graphical design support. Furthermore, we want to express our gratitute to the three anonymous reviewers and the editor for their insightful comments and suggestions that improved the manuscript. This work was partially funded by the Spanish Ministerio de Economia y Competitividad (MINECO) through project AGL2014-55616-C3-3-R and by the UJI through project UJI-B2017-24. T. Pina was the recipient of a postdoctoral grant (PICD) from UJI, and M. Cabedo-López holds a PhD grant from MINECO (grant FPI BES-2015-074570). Funding agencies have no role in the design or analysis of the experiments. All authors of this manuscript declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations'' (in Publisher's Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pina, T., Sabater-Muñoz, B., Cabedo-López, M. et al. Molecular characterization of Cardinium, Rickettsia, Spiroplasma and Wolbachia in mite species from citrus orchards. Exp Appl Acarol 81, 335–355 (2020). https://doi.org/10.1007/s10493-020-00508-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00508-z