Abstract
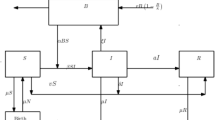
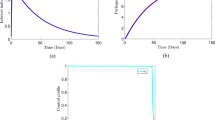
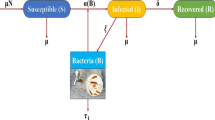
It is often impossible to measure all states affecting spread of a disease. In cholera, asymptomatic and cholera pathogen densities are not practically measurable despite playing a big role in its transmission. They are referred to as inaccessible states of the model and can only be manipulated using the measurable states of the given model. Our interest lies in estimating such states and the parameters catalyzing the spread. A mathematical model for cholera dynamics consisting of five compartments (susceptible, symptomatic, asymptomatic, recovered and bacteria population) with a minimum infection dose (MID) is considered. A method based on observer (from modern control theory) is proposed to estimate the state variables not accessible to measurement and the time-dependent parameters from real data of Senegal. We suppose that the total population of Senegal, the monthly reported cholera-induced deaths and the monthly recovered individuals are known inputs obtainable from real data, and the monthly new cholera cases the system output. An auxiliary system is used, an observer whose solutions converge exponentially to those of an original system and solely utilize known inputs and output of the model. Thus, the estimation of the unmeasured state variables like the pathogen density and the yearly asymptomatic population within the human community playing an important role in the spread of cholera is possible. We derive the expressions for time-dependent infection rate, induced cholera death rate and symptomatic recovery rate and their estimations done using real data. Numerical simulations are then performed for the validation of estimation results. The system together with the observer designed is detectable but is not observable. The observer delivered estimates reflect a close trend already ascertained by other researchers. They indicate the existence of bacteria and asymptomatic individuals in the population of Senegal at any single time for the duration of collection of the data. The ever existence of cholera pathogen explains the endemicity of Cholera in Senegal and other sub-Saharan-African countries owing to role played by the asymptomatic individual in the bacteria density. As such, the heath authorities in Senegal need to educate the general public on hygiene irrespective of observable symptoms to lower the possible number of new infections. We have analytically showed and numerically confirmed the exponential convergence to zero of the estimation errors resulting from the observer model hence the high quality of the estimates.









Similar content being viewed by others
References
Andrews JR, Basu S (2011) Transmission dynamics and control of cholera in haiti: an epidemic model. Lancet 377(9773):1248–1255
Aronna MS, Bliman PA (2017) Interval observer for uncertain time varying sir SI model of vector borne disease
Bauerfeind R, von Graevenitz A, Kimmig P, Schiefer HG, Schwarz T, Slenczka W, Zahner H et al (2016) Zoonoses: infectious diseases transmissible from animals and humans. American Society for Microbiology (ASM)
Bayleyegn YN (2009) Mathematical analysis of a model of cholera transmission dynamics. PhD thesis, University of Manitoba, Canada
Bichara D, Cozic N, Iggidr A (2012) On the estimation of sequestered parasite population in falciparum malaria patients. PhD thesis, INRIA
Bliman P-A, Barros BD (2016) Interval observers for sir epidemic models subject to uncertain seasonality. In: International symposium on positive systems. Springer, pp 31–39
Bliman PA, Efimo D, Ushirobir R (2018) A class of nonlinear adaptive observers for sir epidemic model. In: 2018 Europe control conference (ECC). IEEE, pp 1–6
Bowong S, Kurths J (2010) Parameter estimation based synchronization for an epidemic model with application to tuberculosis in Cameroon. Phys Lett A 374(44):4496–4505
Bowong S, Mountaga L, Bah A, Tewa JJ, Kurths J (2016) Parameter and state estimation in a neisseria meningitidis model: a study case of Niger. Chaos 26(12):123115
Bronze MS, Greenfield RA (2005) Biodefence principles and pathogens horizon bioscience. Horizon Bioscience
CDC. https://www.cdc.gov/cholera/general/. Accessed 21 May 2017
Chao DL, Halloran ME, Longini IM (2011) Vaccination strategies for epidemic cholera in haiti with implications for the developing world. Proc Natl Acad Sci 108(17):7081–7085
Codeço CT (2001) Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect Dis 1(1):1
Colwell RR, Huq A (1994) Environmental reservoir of Vibrio cholerae the causative agent of choleraa. Ann N Y Acad Sci 740(1):44–54
David GL (1979) Introduction to dynamic systems: theory, models and applications
Diaby M, Iggidr A, Sy M (2015) Observer design for a schistosomiasis model. Math Biosci 269:17–29
Frerichs RR. http://www.ph.ucla.edu/epi/snow/firstdiscoveredcholera.html. Accessed 21 Mar 2017
Janeway C, Murphy KP, Travers P, Walport M (2008) Janeway’s immunobiology. Garland Science
Joh RI, Wang H, Weiss H, Weitz JS (2009) Dynamics of indirectly transmitted infectious diseases with immunological threshold. Bull Math Biol 71(4):845–862
King AA, Ionides EL, Pascual M, Bouma MJ (2008) Inapparent infections and cholera dynamics. Nature 454(7206):877–880
Lehr JH, Keeley J et al (2005) Water encyclopedia: domestic, municipal, and industrial water supply and waste disposal. Wiley, New York
Luo J, Wang J, Wang H (2017) Seasonal forcing and exponential threshold incidence in cholera dynamics. Discrete Contin Dyn Syst Ser B 22(6):2017
Miller Neilan RL, Schaefer E, Gaff H, Fister KR, Lenhart S (2010) Modeling optimal intervention strategies for cholera. Bull Math Biol 72(8):2004–2018
Mukandavire Z, Liao S, Wang J, Gaff H, Smith DL, Morris JG (2011) Estimating the reproductive numbers for the 2008–2009 cholera outbreaks in Zimbabwe. Proc Natl Acad Sci 108(21):8767–8772
Nelson EJ, Harris JB, Morris JG Jr, Calderwood SB, Camilli A (2009) Cholera transmission: the host, pathogen and bacteriophage dynamic. Nat Rev Microbiol 7(10):693
Omondi EO, Nyabadza F, Bonyah E, Badu K (2017) Modeling the infection dynamics of onchocerciasis and its treatment. J Biol Syst 25(02):247–277
World Health Organization. http://www.who.int/cholera/countries/en/ Accessed 19 Mar 2017
World Health Organization. http://www.who.int/mediacentre/factsheets/fs107/en/. Accessed 21 Mar 2017
Van de Linde PAM, Forbes GI (1965) Observations on the spread of cholera in Hong Kong, 1961–63. Bull World Health Organ 32(4):515
Wang X, Wang J (2015) Analysis of cholera epidemics with bacterial growth and spatial movement. J Biol Dyn 9(sup1):233–261
Wu H, Zhu H, Miao H, Perelson AS (2008) Parameter identifiability and estimation of HIV/AIDS dynamic models. Bull Math Biol 70(3):785–799
Acknowledgements
BOO and WAW are grateful to Prof. Samuel Bowong for the guidance in writing this paper and for providing the cholera historical data of Senegal. BOO and WAW would also like to thank Prof. Mama Foupouagnigni, the centre president, and the entire AIMS-Cameroon stakeholders for the scholarship opportunity resulting in this paper. EOO acknowledges, with thanks, the support of Strathmore University, Institute of Mathematical Sciences.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ogola, B.O., Woldegerima, W.A. & Omondi, E.O. Parameter and State Estimation in a Cholera Model with Threshold Immunology: A Case Study of Senegal. Bull Math Biol 82, 72 (2020). https://doi.org/10.1007/s11538-020-00755-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11538-020-00755-6