Abstract
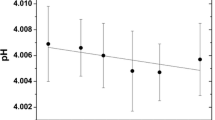
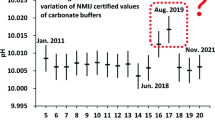
This work presents the results from stability studies for a series of pH-certified reference materials submitted to repeated use conditions (repeated sampling) to meet the requirements from the latest versions of ISO 17034 and ISO Guide 35. The study was applied to reference materials with nominal pH values of 1.68, 4.00, 6.86, 9.18, and 10.01, which were submitted to a repeated use simulation under controlled conditions to reproduce their routine use in analytical laboratories. The materials were analyzed once a month by the primary pH measurement method to check for deviations, trends, or significant variations in the results, using linear regression and normalized error statistical tests. The results indicated that the pH variations found for the materials with pH values of 1.68, 4.00, and 6.86 could be considered normal or statistically insignificant, not affecting their certified values for the time studied. For the materials with pH values of 9.18 and 10.01, the pH variations found were statistically significant and even higher than those from the stability monitoring (bottles not submitted to the repeated use simulation), making it possible to estimate a new associated uncertainty source to the certified value and increase the reliability in the use of these materials.

Graphical abstract



Similar content being viewed by others
References
Olivares IRB, Souza GB, Nogueira ARA, Toledo GTK, Marcki DC. Trends in developments of certified reference materials for chemical analysis - focus on food, water, soil, and sediment matrices. TrAC Trends Anal Chem. 2018;100:53–64.
Bates R. Determination of pH: theory and practice. 1st ed. New York: Wiley; 1964.
Buck RP, Rondinini S, Covington AK, Baucke FGK, Brett CMA, Camoes MF, et al. Measurement of pH. Definition, standards, and procedures (IUPAC recommendations 2002). Pure Appl Chem. 2002;74:2169–200.
Baucke FGK. New IUPAC recommendations on the measurement of pH - background and essentials. Anal Bioanal Chem. 2002;374:772–7.
de Levie R. A pH centenary. Electrochim Acta. 2014;135:604–39.
Pratt KW. Longitudinal meta-analysis of NIST pH standard reference materials®: a complement to pH key comparisons. Anal Bioanal Chem. 2015;407:3193–203.
Gonzaga FB, Dias JC. Long-term stability monitoring of pH reference materials using primary pH method. Anal Bioanal Chem. 2015;407:3249–52.
ISO Guide 35 - reference materials - guidance for characterization and assessment of homogeneity and stability. 4th ed: International Organization for Standardization; 2017.
ISO 17034 - general requirements for the competence of reference material producers. 1st ed: International Organization for Standardization; 2016.
Zakaria O, Rezali MF. Reference materials as a crucial tools for validation and verification of the analytical process. Procedia Soc Behav Sci. 2014;121:204–13.
4500-H+ pH value standard methods for the examination of water and wastewater. 23th ed: American Public Health Association; 2017.
ASTM D6423-19 standard test method for determination of pHe of denatured fuel ethanol and ethanol fuel blends: ASTM International; 2019.
Baucke FGK. Lower temperature limit of NBS (DIN) pH standard buffer solution potassium tetroxalate. Electrochim Acta. 1979;24:95–7.
Stoica D, Brewer PJ, Brown RJC, Fisicaro P. Influence of fabrication procedure on the electrochemical performance of Ag/AgCl reference electrodes. Electrochim Acta. 2011;56:10009–15.
Brewer PJ, Leese RJ, Brown RJC. An improved approach for fabricating Ag/AgCl reference electrodes. Electrochim Acta. 2012;71:252–7.
Brewer PJ, Leach AS, Brown RJC. The role of the electrolyte in the fabrication of Ag|AgCl reference electrodes for pH measurement. Electrochim Acta. 2015;161:80–3.
JCGM 100 – evaluation of measurement data – guide to the expression of uncertainty in measurement (ISO/IEC guide 98-3). Joint Committee for Guides in Metrology, 2008.
Funding
This work was supported by the National Council of Technological and Scientific Development (CNPq) (grant numbers 573894/2008-6 and 307771/2015-6) and the São Paulo Research Foundation (FAPESP) (grant number 2008/57808-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pardellas, L.d., Cunha, K.d. & Gonzaga, F.B. Stability studies of pH reference materials under repeated use conditions by the primary measurement method. Anal Bioanal Chem 412, 5307–5314 (2020). https://doi.org/10.1007/s00216-020-02746-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02746-x