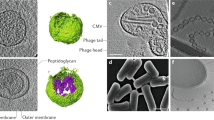
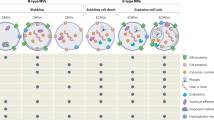
Abstract
Among the major bacterial secretions, outer membrane vesicles (OMVs) are significant and highly functional. The proteins and other biomolecules identified within OMVs provide new insights into the possible functions of OMVs in bacteria. OMVs are rich in proteins, nucleic acids, toxins and virulence factors that play a critical role in bacteria-host interactions. In this review, we discuss some proteins with multifunctional features from bacterial OMVs and their role involving the mechanisms of bacterial survival and defence. Proteins with moonlighting activities in OMVs are discussed based on their functions in bacteria. OMVs harbour many other proteins that are important, such as proteins involved in virulence, defence, and competition. Overall, OMVs are a power-packed aid for bacteria, harbouring many defensive and moonlighting proteins and acting as a survival kit in case of an emergency or as a defence weapon. In summary, OMVs can be defined as bug-out bags for bacterial defence and, therefore, survival.
Similar content being viewed by others
References
Alaniz, R.C., Deatherage, B.L., Lara, J.C., and Cookson, B.T. 2007. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol.179, 7692–7701.
Allocati, N., Masulli, M., Di Ilio, C., and De Laurenzi, V. 2015. Die for the community: an overview of programmed cell death in bacteria. Cell Death Dis.6, e1609.
Amblee, V. and Jeffery, C.J. 2015. Physical features of intracellular proteins that moonlight on the cell surface. PLoS One10, e0130575.
Asally, M., Kittisopikul, M., Rué, P., Du, Y., Hu, Z., Cagatay, T., Robinson, A.B., Lu, H., Garcia-Ojalvo, J., and Süel, G.M. 2012. Localized cell death focuses mechanical forces during 3D patterning in a biofilm. Proc. Natl. Acad. Sci. USA109, 18891–18896.
Bachler, C., Schneider, P., Bahler, P., Lustig, A., and Erni, B. 2005. Escherichia coli dihydroxyacetone kinase controls gene expression by binding to transcription factor DhaR. EMBO J.24, 283–293.
Bai, J., Kim, S.I., Ryu, S., and Yoon, H. 2014. Identification and characterization of outer membrane vesicle-associated proteins in Salmonella enterica serovar typhimurium. Infect. Immun.82, 4001–4010.
Banerjee, S., Nandyala, A.K., Raviprasad, P., Ahmed, N., and Hasnain, S.E. 2007. Iron-dependent RNA-binding activity of Mycobacte-rium tuberculosis aconitase. J. Bacteriol.189, 4046–4052.
Bao, S., Guo, X., Yu, S., Ding, J., Tan, L., Zhang, F., Sun, Y., Qiu, X., Chen, G., and Ding, C. 2014. Mycoplasma synoviae enolase is a plasminogen/fibronectin binding protein. BMC Vet. Res.10, 223.
Barbosa, M.S., Báo, S.N., Andreotti, P.F., de Faria, F.P., Felipe, M.S.S., dos Santos Feitosa, L., Mendes-Giannini, M.J., and Soares, C.M. 2006. Glyceraldehyde-3-phosphate dehydrogenase of Paracocci-dioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect. Immun.74, 382–389.
Biagini, M., Garibaldi, M., Aprea, S., Pezzicoli, A., Doro, F., Beche-relli, M., Taddei, A.R., Tani, C., Tavarini, S., Mora, M.,et al. 2015. The human pathogen Streptococcus pyogenes releases lipoproteins as lipoprotein-rich membrane vesicles. Mol. Cell. Proteomics14, 2138–2149.
Bitto, N.J., Chapman, R., Pidot, S., Costin, A., Lo, C., Choi, J., D’ Cruze, T., Reynolds, E.C., Dashper, S.G., Turnbull, L.,et al. 2017. Bacterial membrane vesicles transport their DNA cargo into host cells. Sci. Rep.7, 7072.
Bonnington, K.E. and Kuehn, M.J. 2014. Protein selection and export via outer membrane vesicles. Biochim. Biophys. Acta1843, 1612–1619.
Boone, T.J. and Tyrrell, G.J. 2012. Identification of the actin and plasminogen binding regions of group B streptococcal phos-phoglycerate kinase. J. Biol. Chem.287, 29035–29044.
Carneiro, C.R., Postol, E., Nomizo, R., Reis, L.F., and Brentani, R.R. 2004. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect.6, 604–608.
Casjens, S. 2003. Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol.49, 277–300.
Charlier, D., Kholti, A., Huysveld, N., Gigot, D., Maes, D., Thia-Toong, T.L., and Glansdorff, N. 2000. Mutational analysis of Escherichia coli PepA, a multifunctional DNA-binding amino-peptidase. J. Mol. Biol.302, 409–424.
Chatterjee, D. and Chaudhuri, K. 2011. Association of cholera toxin with Vibrio cholerae outer membrane vesicles which are internalized by human intestinal epithelial cells. FEBS Lett.585, 1357–1362.
Chen, M.H., Takeda, S., Yamada, H., Ishii, Y., Yamashino, T., and Mizuno, T. 2001. Characterization of the Rcs→YojN→RcsB phosphorelay signaling pathway involved in capsular synthesis in Escherichia coli. Biosci. Biotechnol. Biochem.65, 2364–2367.
Chen, C., Zabad, S., Liu, H., Wang, W., and Jeffery, C. 2018. Moon-Prot 2.0: an expansion and update of the moonlighting proteins database. Nucleic Acids Res.46, D640–D644.
Choi, C.W., Park, E.C., Yun, S.H., Lee, S.Y., Lee, Y.G., Hong, Y., Park, K.R., Kim, S.H., Kim, G.H., and Kim, S.I. 2014. Proteomic characterization of the outer membrane vesicle of Pseudomonas putida KT2440. J. Proteome Res.13, 4298–4309.
Chu, C.H., Liu, M.H., Chen, P.C., Lin, M.H., Li, Y.C., Hsiao, C.D., and Sun, Y.J. 2012. Structures of Helicobacter pylori uridylate kinase: insight into release of the product UDP. Acta Crystallogr. D Biol. Crystallogr.68, 773–783.
Claverys, J.P. and Håvarstein, L.S. 2007. Cannibalism and fratricide: mechanisms and raisons d’être. Nature Rev. Microbiol.5, 219–229.
Corbett, D. and Roberts, I.S. 2008. Capsular polysaccharides in Esche-richia coli. Adv. Appl. Microbiol.65, 1–26.
Dallo, S.F., Kannan, T.R., Blaylock, M.W., and Baseman, J.B. 2002. Elongation factor Tu and E1 ß subunit of pyruvate dehydrogen-ase complex act as fibronectin binding proteins in Mycoplasma pneumoniae. Mol. Microbiol.46, 1041–1051.
Daniely, D., Portnoi, M., Shagan, M., Porgador, A., Givon-Lavi, N., Ling, E., Dagan, R., and Mizrachi Nebenzahl, Y. 2006. Pneumo-coccal 6-phosphogluconate-dehydrogenase, a putative adhesin, induces protective immune response in mice. Clin. Exp. Immunol.144, 254–263.
Das, T., Sehar, S., and Manefield, M. 2013. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep.5, 778–786.
Dashper, S.G., Hendtlass, A., Slakeski, N., Jackson, C., Cross, K.J., Brownfield, L., Hamilton, R., Barr, I., and Reynolds, E.C. 2000. Characterization of a novel outer membrane hemin-binding protein of Porphyromonas gingivalis. J. Bacteriol.182, 6456–6462.
Dillingham, M.S. and Kowalczykowski, S.C. 2008. RecBCD enzyme and the repair of double-stranded DNA breaks. Microbiol. Mol. Biol. Rev.72, 642–671.
Dorward, D.W. and Garon, C.F. 1990. DNA is packaged within membrane-derived vesicles of Gram-negative but not Grampositive bacteria. Appl. Environ. Microbiol.56, 1960–1962.
Dufour, D., Cordova, M., Cvitkovitch, D.G., and Lévesque, C.M. 2011. Regulation of the competence pathway as a novel role associated with a streptococcal bacteriocin. J. Bacteriol.193, 6552–6559.
Dutta, S., Iida, K., Takade, A., Meno, Y., Nair, G.B., and Yoshida, S. 2004. Release of Shiga toxin by membrane vesicles in Shigella dysenteriae serotype 1 strains and in vitro effects of antimicrobials on toxin production and release. Microbiol. Immunol.48, 965–969.
Dwyer, D.J., Camacho, D.M., Kohanski, M.A., Callura, J.M., and Collins, J.J. 2012. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Mol. Cell46, 561–572.
Eddy, J.L., Gielda, L.M., Caulfield, A.J., Rangel, S.M., and Lathem, W.W. 2014. Production of outer membrane vesicles by the plague pathogen Yersinia pestis. PLoS One9, e107002.
Ellis, T.N. and Kuehn, M.J. 2010. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev.74, 81–94.
Ellis, T.N., Leiman, S.A., and Kuehn, M.J. 2010. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun.78, 3822–3831.
Epstein, A.K., Pokroy, B., Seminara, A., and Aizenberg, J. 2011. Bacterial biofilm shows persistent resistance to liquid wetting and gas penetration. Proc. Natl. Acad. Sci. USA108, 995–1000.
Evans, M.R., Fink, R.C., Vazquez-Torres, A., Porwollik, S., Jones-Carson, J., McClelland, M., and Hassan, H.M. 2011. Analysis of the ArcA regulon in anaerobically grown Salmonella enterica sv. Typhimurium. BMC Microbiol.11, 58.
Fedorova, K., Kayumov, A., Woyda, K., Ilinskaja, O., and Forchhammer, K. 2013. Transcription factor TnrA inhibits the bio-synthetic activity of glutamine synthetase in Bacillus subtilis. FEBS Lett.587, 1293–1298.
Freedman, R. 1978. Moonlighting molecules. New Sci.79, 560–561.
Furman, R., Danhart, E.M., NandyMazumdar, M., Yuan, C., Foster, M.P., and Artsimovitch, I. 2015. pH dependence of the stress regulator DksA. PLoS One10, e0120746.
Fushinobu, S., Nishimasu, H., Hattori, D., Song, H.J., and Wakagi, T. 2011. Structural basis for the bifunctionality of fructose-1,6-bisphosphate aldolase/phosphatase. Nature478, 538–541.
Ge, J., Catt, D.M., and Gregory, R.L. 2004. Streptococcus mutans surface a-enolase binds salivary mucin MG2 and human plas-minogen. Infect. Immun.72, 6748–6752.
Gotfredsen, M. and Gerdes, K. 1998. The Escherichia coli relBE genes belong to a new toxin-antitoxin gene family. Mol. Microbiol.29, 1065–1076.
Gottesman, S., Trisler, P., and Torres-Cabassa, A. 1985. Regulation of capsular polysaccharide synthesis in Escherichia coli K-12: characterization of three regulatory genes. J. Bacteriol.162, 1111–1119.
Gunka, K., Newman, J.A., Commichau, F.M., Herzberg, C., Rodri-gues, C., Hewitt, L., Lewis, R.J., and Stulke, J. 2010. Functional dissection of a trigger enzyme: Mutations of the Bacillus subtilis glutamate dehydrogenase RocG that affect differentially its catalytic activity and regulatory properties. J. Mol. Biol.400, 815–827.
Hajishengallis, G., Darveau, R.P., and Curtis, M.A. 2012. The keystone-pathogen hypothesis. Nat. Rev. Microbiol.10, 717–725.
Hardy, C.D. and Cozzarelli, N.R. 2005. A genetic selection for su-percoiling mutants of Escherichia coli reveals proteins implicated in chromosome structure. Mol. Microbiol.57, 1636–1652.
Hazan, R. and Engelberg-Kulka, H. 2004. Escherichia coli mazEF-mediated cell death as a defense mechanism that inhibits the spread of phage P1. Mol. Genet. Genomics272, 227–234.
Hernández, S., Ferragut, G., Amela, I., Perez-Pons, J., Piñol, J., Mozo-Villarias, A., Cedano, J., and Querol, E. 2014. MultitaskProtDB: a database of multitasking proteins. Nucleic Acids Res.42, D517–D520.
Hickey, T.B., Ziltener, H.J., Speert, D.P., and Stokes, R.W. 2010. My-cobacterium tuberculosis employs Cpn60.2 as an adhesin that binds CD43 on the macrophage surface. Cell. Microbiol.12, 1634–1647.
Hirakawa, H., Nishino, K., Hirata, T., and Yamaguchi, A. 2003. Comprehensive studies of drug resistance mediated by overexpre-ssion of response regulators of two-component signal transduc-tion systems in Escherichia coli. J. Bacteriol.185, 1851–1856.
Holtje, J.V. 1995. From growth to autolysis: the murein hydrolases in Escherichia coli. Arch. Microbiol.164, 243–254.
Holzer, H., Schutt, H., Masek, Z., and Mecke, D. 1968. Regulation of two forms of glutamine synthetase in Escherichia coli by the ammonia content of the growth medium. Proc. Natl. Acad. Sci. USA60, 721–724.
Horstman, A.L. and Kuehn, M.J. 2000. Enterotoxigenic Escherichia coli secretes active heat-labile enterotoxin via outer membrane vesicles. J. Biol. Chem.275, 12489–12496.
Huang, Y.H., Ferrières, L., and Clarke, D.J. 2006. The role of the Rcs phosphorelay in Enterobacteriaceae. Res. Microbiol.157, 206–212.
Huberts, D.H. and van der Klei, I.J. 2010. Moonlighting proteins: an intriguing mode of multitasking. Biochim. Biophys. Acta1803, 520–525.
Huntley, J.F., Conley, P.G., Hagman, K.E., and Norgard, M.V. 2007. Characterization of Francisella tularensis outer membrane proteins. J. Bacteriol.189, 561–574.
James, E.S. and Cronan, J.E. 2004. Expression of two Escherichia coli acetyl-CoA carboxylase subunits is autoregulated. J. Biol. Chem.279, 2520–2527.
Jan, A.T. 2017. Outer membrane vesicles (OMVs) of Gram-negative bacteria: a perspective update. Front. Microbiol.8, 1053.
Jeffery, C.J. 2014. An introduction to protein moonlighting. Biochem. Soc. Trans.42, 1679–1683.
Jiang, F., An, C., Bao, Y., Zhao, X., Jernigan, R.L., Lithio, A., Nettleton, D., Li, L., Wurtele, E.S., Nolan, L.K.,et al. 2015. ArcA controls metabolism, chemotaxis, and motility contributing to the patho-genicity of avian pathogenic Escherichia coli. Infect. Immun.83, 3545–3554.
Jin, H., Song, Y.P., Boel, G., Kochar, J., and Pancholi, V. 2005. Group A streptococcal surface GAPDH, SDH, recognizes uPAR/CD87 as its receptor on the human pharyngeal cell and mediates bacterial adherence to host cells. J. Mol. Biol.350, 27–41.
Jones, P.G., Mitta, M., Kim, Y., Jiang, W., and Inouye, M. 1996. Cold shock induces a major ribosomal-associated protein that unwinds double-stranded RNA in Escherichia coli. Proc. Natl. Acad. Sci. USA93, 76–80.
Josephson, B.L. and Fraenkel, D.G. 1974. Sugar metabolism in trans-ketolase mutants of Escherichia coli. J. Bacteriol.118, 1082–1089.
Kadurugamuwa, J.L. and Beveridge, T.J. 1995. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J. Bacteriol.177, 3998–4008.
Kalra, H., Simpson, R.J., Ji, H., Aikawa, E., Altevogt, P., Askenase, P., Bond, V.C., Borràs, F.E., Breakefield, X., Budnik, V.,et al. 2012. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol.10, e1001450.
Kang, Y., Goo, E., Kim, J., and Hwang, I. 2017. Critical role of quorum sensing-dependent glutamate metabolism in homeostatic osmolality and outer membrane vesiculation in Burkholderia glumae. Sci. Rep.7, 44195.
Kato, S., Kowashi, Y., and Demuth, D.R. 2002. Outer membranelike vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog.32, 1–13.
Kesimer, M., Kiliç, N., Mehrotra, R., Thornton, D.J., and Sheehan, J.K. 2009. Identification of salivary mucin MUC7 binding proteins from Streptococcus gordonii. BMC Microbiol.9, 163.
Kesty, N.C., Mason, K.M., Reedy, M., Miller, S.E., and Kuehn, M.J. 2004. Enterotoxigenic Escherichia coli vesicles target toxin delivery into mammalian cells. EMBO J.23, 4538–4549.
Kharina, A., Podolich, O., Faidiuk, I., Zaika, S., Haidak, A., Kukha-renko, O., Zaets, I., Tovkach, F., Reva, O., Kremenskoy, M.,et al. 2015. Temperate bacteriophages collected by outer membrane vesicles in Komagataeibacter intermedius. J. Basic Microbiol.55, 509–513.
Kim, D.K., Lee, J., Kim, S.R., Choi, D.S., Yoon, Y.J., Kim, J.H., Go, G., Nhung, D., Hong, K., Jang, S.C.,et al. 2015. EVpedia: a community web portal for extracellular vesicles research. Bioinfor-matics31, 933–939.
Kim, J.S., Song, S., Lee, M., Lee, S., Lee, K., and Ha, N.C. 2016. Crystal structure of a soluble fragment of the membrane fusion protein HlyD in a type I secretion system of Gram-negative bacteria. Structure24, 477–485.
Kinhikar, A.G., Vargas, D., Li, H., Mahaffey, S.B., Hinds, L., Belisle, J.T., and Laal, S. 2006. Mycobacterium tuberculosis malate syn-thase is a laminin-binding adhesin. Mol. Microbiol.60, 999–1013.
Kinnby, B., Booth, N.A., and Svensäter, G. 2008. Plasminogen binding by oral streptococci from dental plaque and inflammatory lesions. Microbiology154, 924–931.
Kitahara, K. and Suzuki, T. 2009. The ordered transcription of RNA domains is not essential for ribosome biogenesis in Escherichia coli. Mol. Cell34, 760–766.
Kuehn, M.J. and Kesty, N.C. 2005. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev.19, 2645–2655.
Kuo, C.J., Bell, H., Hsieh, C.L., Ptak, C.P., and Chang, Y.F. 2012. Novel mycobacteria antigen 85 complex binding motif on fibron-ectin. J. Biol. Chem.287, 1892–1902.
Kupper, M., Gupta, S.K., Feldhaar, H., and Gross, R. 2014. Versatile roles of the chaperonin GroEL in microorganism-insect interactions. FEMS Microbiol. Lett.353, 1–10.
Le, H.T., Gautier, V., Kthiri, F., Malki, A., Messaoudi, N., Mihoub, M., Landoulsi, A., An, Y.J., Cha, S.S., and Richarme, G. 2012. YajL, prokaryotic homolog of parkinsonism-associated protein DJ-1, functions as a covalent chaperone for thiol proteome. J. Biol. Chem.287, 5861–5870.
Lee, E.Y., Choi, D.S., Kim, K.P., and Gho, Y.S. 2008. Proteomics in Gram-negative bacterial outer membrane vesicles. Mass Spectrom. Rev.27, 535–555.
Lewis, K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev.64, 503–514.
Li, S.J. and Cronan, J.E.Jr. 1993. Growth rate regulation of Escheri-chia coli acetyl coenzyme A carboxylase, which catalyzes the first committed step of lipid biosynthesis. J. Bacteriol.175, 332–340.
Li, W., Rao, D.K., and Kaur, P. 2013. Dual role of the metallopro-tease FtsH in biogenesis of the DrrAB drug transporter. J. Biol. Chem.288, 11854–11864.
Lim, S. and Yoon, H. 2015. Roles of outer membrane vesicles (OMVs) in bacterial virulence. J. Bacteriol. Virol.45, 1–10.
Lin, H., Ye, C., Chen, S., Zhang, S., and Yu, X. 2017. Viable but non-culturable E. coli induced by low level chlorination have higher persistence to antibiotics than their culturable counterparts. Environ. Pollut.230, 242–249.
Loeb, M.R. and Kilner, J. 1978. Release of a special fraction of the outer membrane from both growing and phage T4-infected Esche-richia coli B. Biochim. Biophys. Acta514, 117–127.
MacDonald, I.A. and Kuehn, M.J. 2013. Stress-induced outer membrane vesicle production by Pseudomonas aeruginosa. J. Bac-teriol.195, 2971–2981.
Manning, A.J. and Kuehn, M.J. 2011. Contribution of bacterial outer membrane vesicles to innate bacterial defense. BMC Microbiol.11, 258.
Marcos, C.M., de Oliveira, H.C., da Silva Jde, F., Assato, P.A., Fusco-Almeida, A.M., and Mendes-Giannini, M.J. 2014. The multi-faceted roles of metabolic enzymes in the Paracoccidioides species complex. Front. Microbiol.5, 719.
Maredia, R., Devineni, N., Lentz, P., Dallo, S.F., Yu, J., Guentzel, N., Chambers, J., Arulanandam, B., Haskins, W.E., and Weitao, T. 2012. Vesiculation from Pseudomonas aeruginosa under SOS. Sci. World J.2012, 402919.
Mashburn-Warren, L., Howe, J., Brandenburg, K., and Whiteley, M. 2009. Structural requirements of the Pseudomonas quinolone signal for membrane vesicle stimulation. J. Bacteriol.191, 3411–3414.
McBroom, A.J. and Kuehn, M.J. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol.63, 545–558.
McCaig, W.D., Koller, A., and Thanassi, D.G. 2013. Production of outer membrane vesicles and outer membrane tubes by Francisella novicida. J. Bacteriol.195, 1120–1132.
Melton-Celsa, A.R. 2014. Shiga toxin (Stx) classification, structure, and function. Microbiol. Spectr.2, EHEC-0024-2013.
Menestrina, G., Moser, C., Pellet, S., and Welch, R. 1994. Pore-formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology87, 249–267.
Metzstein, M.M., Stanfield, G.M., and Horvitz, H.R. 1998. Genetics of programmed cell death in C. elegans: past, present and future. Trends Genet.14, 410–416.
Modun, B. and Williams, P. 1999. The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate de-hydrogenase. Infect. Immun.67, 1086–1092.
Muro-Pastor, A.M. and Maloy, S. 1995. Proline dehydrogenase activity of the transcriptional repressor PutA is required for induction of the put operon by proline. J. Biol. Chem.270, 9819–9827.
Olsen, I. and Amano, A. 2015. Outer membrane vesicles-offensive weapons or good Samaritans? J. Oral Microbiol.7, 27468.
Park, D.M., Akhtar, M.S., Ansari, A.Z., Landick, R., and Kiley, P.J. 2013. The bacterial response regulator ArcA uses a diverse binding site architecture to regulate carbon oxidation globally. PLoS Genet.9, e1003839.
Park, A.J., Murphy, K., Surette, M.D., Bandoro, C., Krieger, J.R., Taylor, P., and Khursigara, C.M. 2015. Tracking the dynamic relationship between cellular systems and extracellular subpro-teomes in Pseudomonas aeruginosa biofilms. J. Proteome Res.14, 4524–4537.
Peist, R., Koch, A., Bolek, P., Sewitz, S., Kolbus, T., and Boos, W. 1997. Characterization of the aes gene of Escherichia coli encoding an enzyme with esterase activity. J. Bacteriol.179, 7679–7686.
Perego, M. and Brannigan, J.A. 2001. Pentapeptide regulation of aspartyl-phosphate phosphatases. Peptides22, 1541–1547.
Pierson, T., Matrakas, D., Taylor, Y.U., Manyam, G., Morozov, V.N., Zhou, W., and, van Hoek, M.L. 2011. Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine. J. Proteome Res.10, 954–967.
Pletzer, D., Wolfmeier, H., Bains, M., and Hancock, R.E.W. 2017. Synthetic peptides to target stringent response-controlled virulence in a Pseudomonas aeruginosa murine cutaneous infection model. Front. Microbiol.8, 1867.
Qin, Z., Ou, Y., Yang, L., Zhu, Y., Tolker-Nielsen, T., Molin, S., and Qu, D. 2007. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology153, 2083–2092.
Raffaelli, N., Lorenzi, T., Mariani, P.L., Emanuelli, M., Amici, A., Ruggieri, S., and Magni, G. 1999. The Escherichia coli NadR regulator is endowed with nicotinamide mononucleotide adenylyl-transferase activity. J. Bacteriol.181, 5509–5511.
Raskin, D.M., Judson, N., and Mekalanos, J.J. 2007. Regulation of the stringent response is the essential function of the conserved bacterial G protein CgtA in Vibrio cholerae. Proc. Natl. Acad. Sci. USA104, 4636–4641.
Resch, A., Vecerek, B., Palavra, K., and Bläsi, U. 2010. Requirement of the CsdA DEAD-box helicase for low temperature riboregu-lation of rpoS mRNA. RNA Biol.7, 796–802.
Reyes-Robles, T., Dillard, R.S., Cairns, L.S., Silva-Valenzuela, C.A., Housman, M., Ali, A., Wright, E.R., and Camilli, A. 2018. Vibrio cholerae outer membrane vesicles inhibit bacteriophage infection. J. Bacteriol.200, e00792–17.
Ribet, D. and Cossart, P. 2015. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect.17, 173–183.
Rohmer, L., Hocquet, D., and Miller, S.I. 2011. Are pathogenic bacteria just looking for food? Metabolism and microbial patho-genesis. Trends Microbiol.19, 341–348.
Romanovskaia, A.A. and Nikandrov, V.N. 2007. [Effects of plasmi-nogen, streptokinase and their equimolar complexes with pyruvate kinase on the human neuroblastoma IMR-32 cells]. Tsitologiia49, 656–663.
Romeo, T., Gong, M., Liu, M.Y., and Brun-Zinkernagel, A.M. 1993. Identification and molecular characterization of csrA, a pleiotropic gene from Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties. J. Bacteriol.175, 4744–4755.
Schuch, R. and Maurelli, A.T. 1999. The Mxi-Spa type III secretory pathway of Shigella flexneri requires an outer membrane lip-oprotein, MxiM, for invasin translocation. Infect. Immun.67, 1982–1991.
Schwechheimer, C. and Kuehn, M.J. 2013. Synthetic effect between envelope stress and lack of outer membrane vesicle production in Escherichia coli. J. Bacteriol.195, 4161–4173.
Sengupta, S., Ghosh, S., and Nagaraja, V. 2008. Moonlighting function of glutamate racemase from Mycobacterium tuberculosis: racemization and DNA gyrase inhibition are two independent activities of the enzyme. Microbiology154, 2796–2803.
Shams, F., Oldfield, N.J., Lai, S.K., Tunio, S.A., Wooldridge, K.G., and Turner, D.P. 2016. Fructose-1,6-bisphosphate aldolase of Neisseria meningitidis binds human plasminogen via its C-terminal lysine residue. MicrobiologyOpen5, 340–350.
Sharpe, S.W., Kuehn, M.J., and Mason, K.M. 2011. Elicitation of epithelial cell-derived immune effectors by outer membrane vesicles of nontypeable Haemophilus influenzae. Infect. Immun.79, 4361–4369.
Shendelman, S., Jonason, A., Martinat, C., Leete, T., and Abeliovich, A. 2004. DJ-1 is a redox-dependent molecular chaperone that inhibits a-synuclein aggregate formation. PLoS Biol.2, e362.
Simmons, L.A., Foti, J.J., Cohen, S.E., and Walker, G.C. 2008. The SOS regulatory network. EcoSal Plus2008, 10.1128/ecosalplus.-5.4.3.
Snyder, L. 1995. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol. Microbiol.15, 415–420.
Soler, N., Krupovic, M., Marguet, E., and Forterre, P. 2015. Membrane vesicles in natural environments: a major challenge in viral ecology. ISME J.9, 793–796.
Stout, V., Torres-Cabassa, A., Maurizi, M.R., Gutnick, D., and Got-tesman, S. 1991. RcsA, an unstable positive regulator of capsular polysaccharide synthesis. J. Bacteriol.173, 1738–1747.
Tame, J.R. 2011. Autotransporter protein secretion. Biomol. Concepts2, 525–536.
Tanous, C., Soutourina, O., Raynal, B., Hullo, M.F., Mervelet, P., Gilles, A.M., Noirot, P., Danchin, A., England, P., and Martin-Verstraete, I. 2008. The CymR regulator in complex with the enzyme CysK controls cysteine metabolism in Bacillus subtilis. J. Biol. Chem.283, 35551–35560.
Toledo, A., Coleman, J., Kuhlow, C., Crowley, J., and Benach, J. 2012. The enolase of Borrelia burgdorferi is a plasminogen receptor released in outer membrane vesicles. Infect. Immun.80, 359–368.
Tunio, S.A., Oldfield, N.J., Ala’Aldeen, D.A., Wooldridge, K.G., and Turner, D.P. 2010a. The role of glyceraldehyde 3-phosphate de-hydrogenase (GapA-1) in Neisseria meningitidis adherence to human cells. BMC Microbiol.10, 280.
Tunio, S.A., Oldfield, N.J., Berry, A., Ala’Aldeen, D.A., Wooldridge, K.G., and Turner, D.P. 2010b. The moonlighting protein fruc-tose-1, 6-bisphosphate aldolase of Neisseria meningitidis: surface localization and role in host cell adhesion. Mol. Microbiol.76, 605–615.
Turner, L., Bitto, N.J., Steer, D.L., Lo, C., D’ Costa, K., Ramm, G., Shambrook, M., Hill, A.F., Ferrero, R.L., and Kaparakis-Liaskos, M. 2018. Helicobacter pylori outer membrane vesicle size determines their mechanisms of host cell entry and protein content. Front. Immunol.9, 1466.
Turner, R.J., Bonner, E.R., Grabner, G.K., and Switzer, R.L. 1998. Purification and characterization of Bacillus subtilis PyrR, a bi-functional pyr mRNA-binding attenuation protein/uracil phos-phoribosyltransferase. J. Biol. Chem.273, 5932–5938.
Vartikar, J.V. and Draper, D.E. 1989. S4-16 S ribosomal RNA complex. Binding constant measurements and specific recognition of a 460-nucleotide region. J. Mol. Biol.209, 221–234.
Veith, P.D., Luong, C., Tan, K.H., Dashper, S.G., and Reynolds, E.C. 2018. Outer membrane vesicle proteome of Porphyromonas gin-givalis is differentially modulated relative to the outer membrane in response to heme availability. J. Proteome Res.17, 2377–2389.
Vollmer, W., Joris, B., Charlier, P., and Foster, S. 2008. Bacterial pep-tidoglycan (murein) hydrolases. FEMS Microbiol. Rev.32, 259–286.
Walter, S. 2002. Structure and function of the GroE chaperone. Cell. Mol. Life Sci.59, 1589–1597.
Wampler, J.L., Kim, K.P., Jaradat, Z., and Bhunia, A.K. 2004. Heat shock protein 60 acts as a receptor for the Listeria adhesion protein in Caco-2 cells. Infect. Immun.72, 931–936.
Weilbacher, T., Suzuki, K., Dubey, A.K., Wang, X., Gudapaty, S., Morozov, I., Baker, C.S., Georgellis, D., Babitzke, P., and Romeo, T. 2003. A novel sRNA component of the carbon storage regulatory system of Escherichia coli. Mol. Microbiol.48, 657–670.
Werkman, C.H. 1939. Bacterial dissimilation of carbohydrates. Bac-teriol. Rev.3, 187–227.
Whitworth, D.E. and Morgan, B.H. 2015. Synergism between bacterial GAPDH and OMVs: disparate mechanisms but co-operative action. Front. Microbiol.6, 1231.
Widjaja, M., Harvey, K.L., Hagemann, L., Berry, I.J., Jarocki, V.M., Raymond, B.B.A., Tacchi, J.L., Gründel, A., Steele, J.R., Padula, M.P.,et al. 2017. Elongation factor Tu is a multifunctional and processed moonlighting protein. Sci. Rep.7, 11227.
Woolwine, S.C. and Wozniak, D.J. 1999. Identification of an Esche-richia coli pepA homolog and its involvement in suppression of the algB phenotype in mucoid Pseudomonas aeruginosa. J. Bac-teriol.181, 107–116.
Zakharzhevskaya, N.B., Vanyushkina, A.A., Altukhov, I.A., Shavarda, A.L., Butenko, I.O., Rakitina, D.V., Nikitina, A.S., Manolov, A.I., Egorova, A.N., Kulikov, E.E.,et al. 2017. Outer membrane vesicles secreted by pathogenic and nonpathogenic Bacteroides fra-gilis represent different metabolic activities. Sci. Rep.7, 5008.
Zimmermann, R.A., Garvin, R.T., and Gorini, L. 1971. Alteration of a 30S ribosomal protein accompanying the ram mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA68, 2263–2267.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dineshkumar, K., Aparna, V., Wu, L. et al. Bacterial bug-out bags: outer membrane vesicles and their proteins and functions. J Microbiol. 58, 531–542 (2020). https://doi.org/10.1007/s12275-020-0026-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12275-020-0026-3