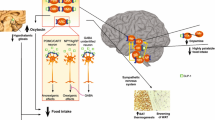
Abstract
Research on energy homeostasis has focused on neuronal signaling; however, the role of glial cells has remained little explored. Glial endozepines exert anorexigenic actions by mechanisms which remain poorly understood. In this context, the present study was designed to decipher the mechanisms underlying the anorexigenic action of endozepines and to investigate their potential curative effect on high-fat diet-induced obesity. We carried out a combination of physiological, pharmacological, and molecular analyses together to dissect the underlying mechanisms of endozepine-induced hypophagia. To evaluate the potential anti-obesity effect of endozepines, different model of obesity were used, i.e., ob/ob and diet-induced obese mice. We show that the intracerebral administration of endozepines enhances satiety by targeting anorexigenic brain circuitry and induces STAT3 phosphorylation, a hallmark of leptin signaling. Strikingly, endozepines are entirely ineffective at reducing food intake in the presence of a circulating leptin antagonist and in leptin-deficient mice (ob/ob) but potentiate the reduced food intake and weight loss induced by exogenous leptin administration in these animals. Endozepines reversed high fat diet-induced obesity by reducing food intake and restored leptin-induced STAT3 phosphorylation in the hypothalamus. Interestingly, we observed that glucose and insulin synergistically enhance tanycytic endozepine expression and release. Finally, endozepines, which induce ERK activation necessary for leptin transport into the brain in cultured tanycytes, require tanycytic leptin receptor expression to promote STAT3 phosphorylation in the hypothalamus. Our data identify endozepines as potential anti-obesity compounds in part through the modulation of the LepR-ERK-dependent tanycytic leptin shuttle.













Similar content being viewed by others
References
Clasadonte J, Prevot V (2018) The special relationship: glia-neuron interactions in the neuroendocrine hypothalamus. Nat Rev Endocrinol 14:25–44. https://doi.org/10.1038/nrendo.2017.124
García-Cáceres C, Balland E, Prevot V, Luquet S, Woods S, Koch M, Horvath T, Yi CX et al (2019) Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat Neurosci 22:7–14. https://doi.org/10.1038/s41593-018-0286-y
Dallaporta M, Bonnet MS, Horner K, Trouslard J, Jean A, Troadec JD (2010) Glial cells of the nucleus tractus solitarius as partners of the dorsal hindbrain regulation of energy balance: a proposal for a working hypothesis. Brain Res 1350:35–42. https://doi.org/10.1016/j.brainres.2010.04.025
Douglass JD, Dorfman MD, Fasnacht R, Shaffer LD, Thaler JP (2017) Astrocyte IKKbeta/NF-kappaB signaling is required for diet-induced obesity and hypothalamic inflammation. Mol Metab 6:366–373. https://doi.org/10.1016/j.molmet.2017.01.010
García-Cáceres C, Fuente-Martín E, Burgos-Ramos E, Granado M, Frago LM, Barrios V, Horvath T, Argente J et al (2011) Differential acute and chronic effects of leptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology. 152:1809–1818. https://doi.org/10.1210/en.2010-1252
Langlet F, Levin BE, Luquet S, Mazzone M, Messina A, Dunn-Meynell AA, Balland E, Lacombe A et al (2013) Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab 17:607–617. https://doi.org/10.1016/j.cmet.2013.03.004
Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA et al (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122:153–162. https://doi.org/10.1172/JCI59660
Zhang Y, Reichel JM, Han C, Zuniga-Hertz J, Cai D (2017) Astrocytic process plasticity and IKKbeta/NF-kappaB in central control of blood glucose, blood pressure and body weight. Cell Metab 25:1091–1102. https://doi.org/10.1016/j.cmet.2017.04.002
Grayson BE, Levasseur PR, Williams SM, Smith MS, Marks DL, Grove KL (2010) Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology. 151:1622–1632. https://doi.org/10.1210/en.2009-1019
García-Cáceres C, Quarta C, Varela L, Gao Y, Gruber T, Legutko B, Jastroch M, Johansson P et al (2016) Astrocytic insulin signaling couples brain glucose uptake with nutrient availability. Cell. 166:867–880. https://doi.org/10.1016/j.cell.2016.07.028
Horvath TL, Sarman B, García-Cáceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J et al (2010) Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A 107:14875–14880. https://doi.org/10.1073/pnas.1004282107
Fuente-Martín E, García-Cáceres C, Granado M, de Ceballos ML, Sánchez-Garrido MÁ, Sarman B, Liu ZW, Dietrich MO et al (2012) Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J. Cli. Invest 122:3900–3913. https://doi.org/10.1172/JCI64102
Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu ZW, Zimmer MR et al (2014) Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat Neurosci 17:908–910. https://doi.org/10.1038/nn.3725
Friedman JM (2014) 20 years of leptin: leptin at 20: an overview. J Endocrinol 223:T1-8. https://doi.org/10.1530/JOE-14-0405
Balland E, Dam J, Langlet F, Caron E, Steculorum S, Messina A, Rasika S, Falluel-Morel A et al (2014) Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab 19:293–301. https://doi.org/10.1016/j.cmet.2013.12.015
Farzampour Z, Reimer RJ, Huguenard J (2015) Endozepines. Adv Pharmacol 72:147–164. https://doi.org/10.1016/bs.apha.2014.10.005
Tonon MC, Vaudry H, Chuquet J, Guillebaud F, Fan J, Masmoudi-Kouki O, Vaudry D, Lanfray D et al (2020) Endozepines and their receptors: structure, functions and pathophysiological significance. Pharmacol Ther 208:107386. https://doi.org/10.1016/j.pharmthera.2019.06.008
Tonon MC, Désy L, Nicolas P, Vaudry H, Pelletier G (1990) Immunocytochemical localization of the endogenous benzodiazepine ligand octadecaneuropeptide (ODN) in the rat brain. Neuropeptides 15:17–24. https://doi.org/10.1016/0143-4179(90)90155-r
Guillebaud F, Girardet C, Abysique A, Gaigé S, Barbouche R, Verneuil J, Jean A, Leprince J et al (2017) Glial endozepines inhibit feeding-related autonomic functions by acting at the brainstem level. Front Neurosci 11:308. https://doi.org/10.3389/fnins.2017.00308
De Mateos-Verchere JG, Leprince J, Tonon MC, Vaudry H, Costentin J (2001) The octadecaneuropeptide [diazepam-binding inhibitor (33-50)] exerts potent anorexigenic effects in rodents. Eur J Pharmacol 414:225–231. https://doi.org/10.1016/s0014-2999(01)00771-3
Matsuda K, Wada K, Miura T, Maruyama K, Shimakura SI, Uchiyama M, Leprince J, Tonon MC et al (2007) Effect of the diazepam-binding inhibitor-derived peptide, octadecaneuropeptide, on food intake in goldfish. Neuroscience. 150:425–432. https://doi.org/10.1016/j.neuroscience.2007.09.012
Lanfray D, Arthaud S, Ouellet J, Compère V, Do Rego JL, Leprince J, Lefranc B, Castel H et al (2013) Gliotransmission and brain glucose sensing: Critical role of endozepines. Diabetes 62:801–810. https://doi.org/10.2337/db11-0785
do Rego JC, Orta MH, Leprince J, Tonon MC, Vaudry H, Costentin J (2007) Pharmacological characterization of the receptor mediating the anorexigenic action of the octadecaneuropeptide: evidence for an endozepinergic tone regulating food intake. Neuropsychopharmacology 32:1641–1648. https://doi.org/10.1038/sj.npp.1301280
Compère V, Li S, Leprince J, Tonon MC, Vaudry H, Pelletier G (2003) Effect of intracerebroventricular administration of the octadecaneuropeptide on the expression of pro-opiomelanocortin, neuropeptide Y and corticotropin-releasing hormone mRNAs in rat hypothalamus. J Neuroendocrinol 15:197–203. https://doi.org/10.1046/j.1365-2826.2003.00970.x
Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL (2004) Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304:110–115. https://doi.org/10.1126/science.1089459
Leprince J, Gandolfo P, Thoumas JL, Patte C, Fauchère JL, Vaudry H, Tonon MC (1998) Structure-activity relationships of a series of analogues of the octadecaneuropeptide ODN on calcium mobilization in rat astrocytes. J Med Chem 41:4433–4438. https://doi.org/10.1021/jm980275d
Gaigé S, Djelloul M, Tardivel C, Airault C, Félix B, Jean A, Lebrun B, Troadec JD et al (2014) Modification of energy balance induced by the food contaminant T-2 toxin: a multimodal gut-to-brain connection. Brain Behav Immun 37:54–72. https://doi.org/10.1016/j.bbi.2013.12.008
Bellefontaine N, Chachlaki K, Parkash J, Vanacker C, Colledge W, d’Anglemont de Tassigny X, Garthwaite J, Bouret SG et al (2014) Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J. Clin. Invest 124:2550–2559. https://doi.org/10.1172/JCI65928
Girardet C, Bonnet MS, Jdir R, Sadoud M, Thirion S, Tardivel C, Roux J, Lebrun B et al (2016) The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS One 6:e26134. https://doi.org/10.1371/journal.pone.0026134
Dallaporta M, Pecchi E, Pio J, Jean A, Horner KC, Troadec JD (2009) Expression of leptin receptor by glial cells of the nucleus tractus solitarius: possible involvement in energy homeostasis. J Neuroendocrinol 21:57–67. https://doi.org/10.1111/j.1365-2826.2008.01799.x
Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A (2013) Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat. Protoc 8:1149–1154. https://doi.org/10.1038/nprot.2013.055
Andrey P, Maurin Y (2005) Free-D: an integrated environment for three-dimensional reconstruction from serial sections. J Neurosci Methods 45:233–244. https://doi.org/10.1016/j.jneumeth.2005.01.006
Ghouili I, Bahdoudi S, Morin F, Amri F, Hamdi Y, Coly PM, Walet-Balieu ML, Leprince J et al (2018) Endogenous expression of ODN-related peptides in astrocytes contributes to cell protection against oxidative stress: astrocyte-neuron crosstalk relevance for neuronal survival. Mol Neurobiol 55:4596–4611. https://doi.org/10.1007/s12035-017-0630-3
Vaudry H, Tonon MC, Delarue C, Vaillant R, Kraicer J (1978) Biological and radioimmunological evidence for melanocyte stimulating hormones (MSH) of extrapituitary origin in the rat brain. Neuroendocrinology. 27:9–24. https://doi.org/10.1159/000122796
Pecchi E, Dallaporta M, Charrier C, Pio J, Jean A, Moyse E, Troadec JD (2007) Glial fibrillary acidic protein (GFAP)-positive radial-like cells are present in the vicinity of proliferative progenitors in the nucleus tractus solitarius of adult rat. J Comp Neurol 501:353–368. https://doi.org/10.1002/cne.21259
Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B (2013) Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol 521:3389–3405. https://doi.org/10.1002/cne.23355
Hokfelt T, Foster G, Schultzberg M, Meister B, Schalling M, Goldstein M, Hemmings HC Jr, Ouimet C et al (1988) DARPP-32 as a marker for D-1 dopaminoceptive cells in the rat brain: prenatal development and presence in glial elements (tanycytes) in the basal hypothalamus. Adv Exp Med Biol 235:65–82. https://doi.org/10.1007/978-1-4899-2723-1_6
Sidibe A, Mullier A, Chen P, Baroncini M, Boutin JA, Delagrange P, Prevot V, Jockers R (2010) Expression of the orphan GPR50 protein in rodent and human dorsomedial hypothalamus, tanycytes and median eminence. J Pineal Res 48:263–269. https://doi.org/10.1111/j.1600-079X.2010.00750.x
Barrachina MD, Martínez V, Wang L, Wei JY, Taché Y (1997) Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A 94:10455–10460. https://doi.org/10.1073/pnas.94.19.10455
Halpern Z, Elinav E, Gertler A (2011) Development and characterization of high affinity leptins and leptin antagonists. J Biol Chem 286:4429–4442. https://doi.org/10.1074/jbc.M110.196402
Frayling C, Britton R, Dale N (2011) ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol 589:2275–2286. https://doi.org/10.1113/jphysiol.2010.202051
Prevot V, Dehouck B, Sharif A, Ciofi P, Giaconini P, Clasadonte J (2018) The versatile tanycyte: a hypothalamic integrator of reproduction and energy metabolism. Endocr Rev 39:333–368. https://doi.org/10.1210/er.2017-00235
Parkash J, Messina A, Langlet F, Cimino I, Loyens A, Mazur D, Gallet S, Balland E et al (2015) Semaphorin7A regulates neuroglial plasticity in the adult hypothalamic median eminence. Nat Commun 6:6385. https://doi.org/10.1038/ncomms7385
Alho H, Fremeau RT Jr, Tiedge H, Wilcox J, Bovolin P, Brosius J, Roberts JL, Costa E (1988) Diazepam binding inhibitor gene expression: location in brain and peripheral tissues of rat. Proc Natl Acad Sci U S A 85:7018–7022. https://doi.org/10.1073/pnas.85.18.7018
Seeley RJ, Blake K, Rushing PA, Benoit S, Eng J, Woods SC, D’Alessio D (2000) The role of CNS glucagon-like peptide-1 (7-36) amide receptors in mediating the visceral illness effects of lithium chloride. J Neurosci 20:1616–1621. https://doi.org/10.1523/JNEUROSCI.20-04-01616.2000
Yamamoto K, Yamatodani A (2018) Strain differences in the development of cisplatin-induced pica behavior in mice. J Pharmacol Toxicol Methods 91:66–71. https://doi.org/10.1016/j.vascn.2018.01.559
van Swieten MM, Pandit R, Adan RA, van der Plasse G (2014) The neuroanatomical function of leptin in the hypothalamus. J. Chem. Neuroanat 61-62:207–220. https://doi.org/10.1016/j.jchemneu.2014.05.004
Dragunow M, Faull R (1989) The use of c-Fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29:261–265. https://doi.org/10.1016/0304-3940(90)90341-6
Bouyakdan K, Martin H, Liénard F, Budry L, Taib B, Rodaros D, Chrétien C, Biron É et al (2019) The gliotransmitter ACBP controls feeding and energy homeostasis via the melanocortin system. J Clin Invest 130:2417–2430. https://doi.org/10.1172/JCI123454
Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M (2013) Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33:3624–3632. https://doi.org/10.1523/JNEUROSCI.2742-12.2013
Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M et al (2006) Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 443:709–712. https://doi.org/10.1038/nature05162
Bonnet MS, Pecchi E, Trouslard J, Jean A, Dallaporta M, Troadec JD (2009) Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J. Neuroinflammation 6:27. https://doi.org/10.1186/1742-2094-6-27
Shimizu H, Ohsaki A, Oh-I S, Okada S, Mori M (2009) A new anorexigenic protein, nesfatin-1. Peptides 30:995–998. https://doi.org/10.1016/j.peptides.2009.01.002
Katsurada K, Maejima Y, Nakata M, Kodaira M, Suyama S, Iwasaki Y, Kario K, Yada T (2014) Endogenous GLP-1 acts on paraventricular nucleus to suppress feeding: projection from nucleus tractus solitarius and activation of corticotropin-releasing hormone, nesfatin-1 and oxytocin neurons. Biochem Biophys Res Commun 451:276–281. https://doi.org/10.1016/j.bbrc.2014.07.116
Yettefti K, Orsini JC, el Ouazzani T, Himmi T, Boyer A, Perrin J (1995) Sensitivity of nucleus tractus solitarius neurons to induced moderate hyperglycemia, with special reference to catecholaminergic regions. J Auton Nerv Syst 51:191–197. https://doi.org/10.1016/0165-1838(94)00130-c
Gandolfo P, Patte C, Leprince J, Thoumas J-L, Vaudry H, Tonon M-C (1997) The stimulatory effect of the octadecaneuropeptide (ODN) on cytosolic Ca2+ in rat astrocytes is not mediated through classical benzodiazepine receptors. Eur J Pharmacol 322:275–281. https://doi.org/10.1016/s0014-2999(97)00012-5
Lamacz M, Tonon MC, Smih-Rouet F, Patte C, Gasque P, Fontaine M, Vaudry H (1996) The endogenous benzodiazepine receptor ligand ODN increases cytosolic calcium in cultured rat astrocytes. Mol Brain Res 37:290–296. https://doi.org/10.1016/0169-328x(95)00330-u
Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ et al (2003) STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 421:856–859. https://doi.org/10.1038/nature01388
Gao Q, Wolfgang MJ, Neschen S, Morino K, Horvath TL, Shulman GI, Fu XY (2004) Disruption of neural signal transducer and activator of transcription 3 causes obesity, diabetes, infertility, and thermal dysregulation. Proc Natl Acad Sci U S A 101:4661–4666. https://doi.org/10.1073/pnas.0303992101
Lanfray D, Caron A, Roy MC, Laplante M, Morin F, Leprince J, Tonon MC, Richard D (2016) Involvement of the acyl-CoA binding domain containing 7 in the control of food intake and energy expenditure in mice. Elife. 5:e11742. https://doi.org/10.7554/eLife.11742
Myers MG, Cowley MA, Munzberg H (2008) Mechanisms of leptin action and leptin resistance. Annu Rev Physiol 70:537–556. https://doi.org/10.1146/annurev.physiol.70.113006.100707
Caro JF, Kolaczynski JW, Nyce MR, Ohannesian JP, Opentanova I, Goldman WH, Lynn RB, Zhang PL et al (1996) Decreased cerebrospinal-fluid/serum leptin ratio in obesity: a possible mechanism for leptin resistance. Lancet 348:159–161. https://doi.org/10.1016/s0140-6736(96)03173-x
El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS (2000) Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest 105:1827–1832. https://doi.org/10.1016/s0140-6736(96)03173-x
Schwartz MW, Peskind E, Raskind M, Boyko EJ, Porte D Jr (1996) Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nat Med 2:589–593. https://doi.org/10.1038/nm0596-589
Hamdi Y, Kaddour H, Vaudry D, Bahdoudi S, Douiri S, Leprince J, Castel H, Vaudry H et al (2012) The octadecaneuropeptide ODN protects astrocytes against hydrogen peroxide-induced apoptosis via a PKA/MAPK-dependent mechanism. PLoS One 7:e42498. https://doi.org/10.1371/journal.pone.0042498
Maniscalco JW, Rinaman L (2014) Systemic leptin dose-dependently increases STAT3 phosphorylation within hypothalamic and hindbrain nuclei. Am J Phys 306:R576–R585. https://doi.org/10.1152/ajpregu.00017.2014
Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P (1995) Reombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 269:546–549. https://doi.org/10.1126/science.7624778
Balland E, Cowley MA (2015) New insights in leptin resistance mechanisms in mice. Front Neuroendocrinol 39:59–65. https://doi.org/10.1016/j.yfrne.2015.09.004
Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle D, Erickson JC, Palmiter RD, Schwartz MW (1998) Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes. 47:538–543. https://doi.org/10.2337/diabetes.47.4.538
Ottaway N, Mahbod P, Rivero B, Norman LA, Gertler A, D'Alessio DA, Perez-Tilve D (2015) Diet-induced obese mice retain endogenous leptin action. Cell Metab 21:877–882. https://doi.org/10.1016/j.cmet.2015.04.015
Knight ZA, Hannan KS, Greenberg ML, Friedman JM (2010) Hyperleptinemia is required for the development of leptin resistance. PLoS One 5:e11376. https://doi.org/10.1371/journal.pone.0011376
Funding
This work was supported by funding obtained from the Aix-Marseille Université, the Institut National de la Recherche Agronomique (INRA), the Institut National de la Santé et de la Recherche Médicale (Inserm), the Agence Nationale de la Recherche grant Glioshuttle4Metabolim (ANR-15-CE14-0025 to VP), and EZICROM (ANR-16-CE14-0011 to JL, VP, and JDT). The authors acknowledge Dr. S. Rasika for the editing of the manuscript and Coraline Airault, Catherine Tardivel, and Elise Courvoisier for their support in qPCR experiments (plateforme Analyse et Valorisation de la Biodiversité, Marseille). We also thank the Centre Pluridisciplinaire de Microscopie Electronique et de Microanalyse (CP2M, Aix-Marseille Université) for the access to their confocal microscopy equipment.
Author information
Authors and Affiliations
Contributions
FG, MD, MD, CP, GR, KP, SR, DL, SG, MD, and JL performed the experiments. JL contributed to the ODN and OP synthesis. FG, FM, AJ, MCT, SG, BL, MD, JL, VP, and JDT designed the study and analyzed the data. BL, JL, VP, and JDT wrote the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
FG is a fellow of the Nestlé France Foundation. CP is affiliated with Biomeostasis CRO. The other authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Intracerebral administration of endozepines enhances satiety and induces STAT3 phosphorylation in the hypothalamus and brainstem.
• The anorexigenic action of endozepines requires a fully operative leptin signaling pathway.
• Endozepines reverse a diet-induced obese phenotype by reducing food intake.
• Leptin receptor expression into tanycytes is required for endozepine-induced STAT3 phosphorylation.
Rights and permissions
About this article
Cite this article
Guillebaud, F., Duquenne, M., Djelloul, M. et al. Glial Endozepines Reverse High-Fat Diet-Induced Obesity by Enhancing Hypothalamic Response to Peripheral Leptin. Mol Neurobiol 57, 3307–3333 (2020). https://doi.org/10.1007/s12035-020-01944-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01944-z