Abstract
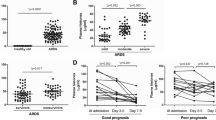
Previous studies showed that extracellular histones could damage organs, but the role of extracellular histones in pneumonia patients with acute kidney injury (AKI) is unknown. This study aims to investigate the impact of extracellular histones on patients with community-acquired pneumonia (CAP) developed AKI. Blood samples were obtained within 24 h after admission to hospital from patients who were diagnosed with CAP. According to the discharge diagnosis, the patients were divided into 2 groups (Non-AKI and AKI). In vitro, A549 cells were treated with lipopolysaccharides (LPS) and conditioned media were collected. HK2 cells were exposed to the conditioned media or not. Cells proliferation and apoptosis of HK2 were determined. Clinically, Log2 Histones (OR 3.068; 95% CI 1.544–6.097, P = 0.001) and estimated glomerular filtration rate (eGFR) (OR 0.945; 95% CI 0.914–0.978, P = 0.001) were predictors of AKI in CAP patients. Compared to the lower histones group, patients in the higher histones group were more likely to be admitted to ICU, receive mechanical ventilation, and have a longer length of in-hospital stay. In vitro, A549 cells injured by LPS released extracellular histones, in conditioned media which significantly promoted HK2 cells apoptosis. Extracellular histones was a high risk factor for developing AKI in CAP patients and a predictor of worse short-term outcomes. We also showed that extracellular histones in conditioned media damaged HK2 cells.
Trial registration number: KY20181102-03; Date of registration: 20181102.




Similar content being viewed by others

References
Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS (2014) Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol 9:12–20. https://doi.org/10.2215/CJN.02730313
Teixeira JP, Ambruso S, Griffin BR, Faubel S (2019) Pulmonary consequences of acute kidney injury. Semin Nephrol 39:3–16. https://doi.org/10.1016/j.semnephrol.2018.10.001
Harrois A, Soyer B, Gauss T, Hamada S, Raux M, Duranteau J (2018) Prevalence and risk factors for acute kidney injury among trauma patients: a multicenter cohort study. Crit Care 22:344. https://doi.org/10.1186/s13054-018-2265-9
Scheel PJ, Liu M, Rabb H (2008) Uremic lung: new insights into a forgotten condition. Kidney Int 74:849–851. https://doi.org/10.1038/ki.2008.390
Thomas CP, Ryan M, Chapman JD, Stason WB, Tompkins CP, Suaya JA, Polsky D, Mannino DM, Shepard DS (2012) Incidence and cost of pneumonia in medicare beneficiaries. Chest 142:973–981. https://doi.org/10.1378/chest.11-1160
Chawla LS, Amdur RL, Faselis C, Li P, Kimmel PL, Palant CE (2017) Impact of acute kidney injury in patients hospitalized with pneumonia. Crit Care Med 45:600–606. https://doi.org/10.1097/CCM.0000000000002245
Marsman G, Zeerleder S, Luken BM (2016) Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis 7:e2518. https://doi.org/10.1038/cddis.2016.410
Xu J, Zhang X, Pelayo R, Monestier M, Ammollo CT, Semeraro F, Taylor FB, Esmon NL, Lupu F, Esmon CT (2009) Extracellular histones are major mediators of death in sepsis. Nat Med 15:1318–1321. https://doi.org/10.1038/nm.2053
Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hagele H, Lichtnekert J, Hagemann JH, Rupanagudi KV, Ryu M, Schwarzenberger C et al (2012) Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol 23:1375–1388. https://doi.org/10.1681/ASN.2011111077
Bosmann M, Grailer JJ, Ruemmler R, Russkamp NF, Zetoune FS, Sarma JV, Standiford TJ, Ward PA (2013) Extracellular histones are essential effectors of C5aR- and C5L2-mediated tissue damage and inflammation in acute lung injury. Faseb j 27:5010–5021. https://doi.org/10.1096/fj.13-236380
Xu J, Zhang X, Monestier M, Esmon NL, Esmon CT (2011) Extracellular histones are mediators of death through TLR2 and TLR4 in mouse fatal liver injury. J Immunol 187:2626–2631. https://doi.org/10.4049/jimmunol.1003930
Singbartl K, Bishop JV, Wen X, Murugan R, Chandra S, Filippi MD, Kellum JA (2011) Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int 80:633–644. https://doi.org/10.1038/ki.2011.201
Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, Angus DC, Kellum JA (2010) Genetic, inflammatory markers of sepsis I: acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 77:527–535. https://doi.org/10.1038/ki.2009.502
Prina E, Ranzani OT, Torres A (2015) Community-acquired pneumonia. Lancet 386:1097–1108. https://doi.org/10.1016/S0140-6736(15)60733-4
Kellum JA, Lameire N (2013) Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 17:204. https://doi.org/10.1186/cc11454
Liu T, Huang W, Szatmary P, Abrams ST, Alhamdi Y, Lin Z, Greenhalf W, Wang G, Sutton R, Toh CH (2017) Accuracy of circulating histones in predicting persistent organ failure and mortality in patients with acute pancreatitis. Br J Surg 104:1215–1225. https://doi.org/10.1002/bjs.10538
Yang R, Zou X, Tenhunen J, Tønnessen TI (2017) HMGB1 and extracellular histones significantly contribute to systemic inflammation and multiple organ failure in acute liver failure. Mediators Inflamm 2017:1–6. https://doi.org/10.1155/2017/5928078
Kasetty G, Papareddy P, Bhongir RKV, Ali MN, Mori M, Wygrecka M, Erjefalt JS, Hultgardh-Nilsson A, Palmberg L, Herwald H, Egesten A (2019) Osteopontin protects against lung injury caused by extracellular histones. Mucosal Immunol 12:39–50. https://doi.org/10.1038/s41385-018-0079-3
Husain-Syed F, Slutsky AS, Ronco C (2016) Lung-kidney cross-talk in the critically Ill patient. Am J Respir Crit Care Med 194:402–414. https://doi.org/10.1164/rccm.201602-0420CP
Nakazawa D, Kumar SV, Marschner J, Desai J, Holderied A, Rath L, Kraft F, Lei Y, Fukasawa Y, Moeckel GW et al (2017) Histones and neutrophil extracellular traps enhance tubular necrosis and remote organ injury in ischemic AKI. J Am Soc Nephrol 28:1753–1768. https://doi.org/10.1681/ASN.2016080925
Ashar HK, Mueller NC, Rudd JM, Snider TA, Achanta M, Prasanthi M, Pulavendran S, Thomas PG, Ramachandran A, Malayer JR et al (2018) The role of extracellular histones in influenza virus pathogenesis. Am J Pathol 188:135–148. https://doi.org/10.1016/j.ajpath.2017.09.014
Kutcher ME, Xu J, Vilardi RF, Ho C, Esmon CT, Cohen MJ (2012) Extracellular histone release in response to traumatic injury: implications for a compensatory role of activated protein C. J Trauma Acute Care Surg 73:1389–1394. https://doi.org/10.1097/TA.0b013e318270d595
Abrams ST, Zhang N, Manson J, Liu T, Dart C, Baluwa F, Wang SS, Brohi K, Kipar A, Yu W et al (2013) Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med 187:160–169. https://doi.org/10.1164/rccm.201206-1037OC
Chen Q, Ye L, Jin Y, Zhang N, Lou T, Qiu Z, Jin Y, Cheng B, Fang X (2012) Circulating nucleosomes as a predictor of sepsis and organ dysfunction in critically ill patients. Int J Infect Dis 16:e558–564. https://doi.org/10.1016/j.ijid.2012.03.007
Saffarzadeh M, Preissner KT (2013) Fighting against the dark side of neutrophil extracellular traps in disease: manoeuvres for host protection. Curr Opin Hematol 20:3–9. https://doi.org/10.1016/j.ijid.2012.03.007
Bosmann M, Ward PA (2014) Protein-based therapies for acute lung injury: targeting neutrophil extracellular traps. Expert Opin Ther Targets 18:703–714. https://doi.org/10.1517/14728222.2014.902938
Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, Lohmeyer J, Preissner KT (2012) Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS ONE 7:e32366. https://doi.org/10.1371/journal.pone.0032366
Zallen G, Moore EE, Johnson JL, Tamura DY, Aiboshi J, Biffl WL, Silliman CC (1999) Circulating postinjury neutrophils are primed for the release of proinflammatory cytokines. J Trauma 46:42–48. https://doi.org/10.1097/00005373-199901000-00007
Acknowledgments
We thank all the staff participating in the study.
Funding
This study was supported by the Specific Project for Technology Clinical Medicine of Jiangsu Province (BL2014015), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX18_0428) and the Research Project of Jiangsu Provincial Commission of Health and Family Planning (BJ15004).
Author information
Authors and Affiliations
Contributions
Study design: Changchun Cao, Xin Wan. Data collection: Yasser Gendoo, Dawei Chen. Data analysis: Min Gao, Mengqing Ma, Wei Shao. Cell culture: Min Gao. Manuscript writing: Min Gao, Binbin Pan.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, M., Wan, X., Ma, M. et al. Kidney injury induced by elevated histones in community-acquired pneumonia. Mol Cell Biochem 471, 155–163 (2020). https://doi.org/10.1007/s11010-020-03775-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-020-03775-x