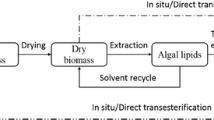
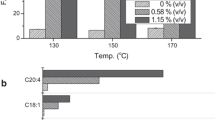
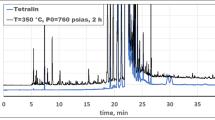
Abstract
Microalgae have been widely considered as a promising alternative to renewable feedstock to biofuel production. Considering the many oleaginous strains of microalgae, this work assessed the potential of two species, Chlorella minutissima and Nannochloropsis gaditana, to be tested as raw material for ethyl esters production of biodiesel value. This work demonstrates an efficient pilot-size photoautotrophic growth of the two strains using 40-L bubble-column photobioreactors, providing lipid productivities of around 15.2 and 8.7 mg L−1 day−1 for C. minutissima and N. gaditana, respectively. The lipid-bearing biomass, which presented fatty acids composition similar to vegetable oils, such as soybean, rich in palmitic, oleic, and linoleic acids, was then assayed in direct trans/esterification reactions using ethanol as acyl donor, lipid extracting and solvent for the liquid phase in a pressurized reactor. The reactions, which were catalyzed by a heterogeneous acid catalyst (12-molybdophosphoric acid supported onto aluminum oxide), demonstrated an efficient route for producing a product mixture containing ester contents greater than 96.5 wt%, total conversion of triacylglycerols, and low levels of mono- and diacylglycerols, promoting an ethyl ester mixture with possible integration within the biodiesel market specifications.


Similar content being viewed by others
References
Zhu L (2015) Biorefinery as a promising approach to promote microalgae industry: an innovative framework. Renew Sust Energ Rev 41:1376–1384. https://doi.org/10.1016/j.rser.2014.09.040
Halfhide T, Åkerstrøm A, Lekang OI, Gislerød HR, Ergas SJ (2014) Production of algal biomass, chlorophyll, starch and lipids using aquaculture wastewater under axenic and non-axenic conditions. Algal Res 6:152–159. https://doi.org/10.1016/j.algal.2014.10.009
Plaza M, Santoyo S, Jaime L, Reina GG-B, Herrero M, Señoráns FJ, Ibáñez E (2010) Screening for bioactive compounds from algae. J Pharm Biomed Anal 51(2):450–455. https://doi.org/10.1016/j.jpba.2009.03.016
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25(3):294–306. https://doi.org/10.1016/j.biotechadv.2007.02.001
Mitra D, van Leeuwen JH, Lamsal B (2012) Heterotrophic/mixotrophic cultivation of oleaginous Chlorella vulgaris on industrial co-products. Algal Res 1(1):40–48. https://doi.org/10.1016/j.algal.2012.03.002
Jinkerson RE, Radakovits R, Posewitz MC (2013) Genomic insights from the oleaginous model alga Nannochloropsis gaditana. Bioengineered 4(1):37–43. https://doi.org/10.4161/bioe.21880
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sust Energ Rev 14(1):217–232. https://doi.org/10.1016/j.rser.2009.07.020
Carvalho AKF, da Conceição LRV, Silva JPV, Perez VH, de Castro HF (2017) Biodiesel production from Mucor circinelloides using ethanol and heteropolyacid in one and two-step transesterification. Fuel 202:503–511. https://doi.org/10.1016/j.fuel.2017.04.063
Rodrigues Reis CE, Bento HBS, Carvalho AKF, Rajendran A, Hu B, De Castro HF (2019) Critical applications of Mucor circinelloides within a biorefinery context. Crit Rev Biotechnol 39(4):555–570. https://doi.org/10.1080/07388551.2019.1592104
Go AW, Sutanto S, Ong LK, Tran-Nguyen PL, Ismadji S, Ju Y-H (2016) Developments in in-situ (trans) esterification for biodiesel production: a critical review. Renew Sust Energ Rev 60:284–305. https://doi.org/10.1016/j.rser.2016.01.070
Loures CCA, Amaral MS, Da Rós PCM, Zorn SMFE, de Castro HF, Silva MB (2018) Simultaneous esterification and transesterification of microbial oil from Chlorella minutissima by acid catalysis route: a comparison between homogeneous and heterogeneous catalysts. Fuel 211:261–268. https://doi.org/10.1016/j.fuel.2017.09.073
Carvalho AKF, Rivaldi JD, Barbosa JC, de Castro HF (2015) Biosynthesis, characterization and enzymatic transesterification of single cell oil of Mucor circinelloides–a sustainable pathway for biofuel production. Bioresour Technol 181:47–53. https://doi.org/10.1016/j.biortech.2014.12.110
da Conceição LRV, Reis CER, de Lima R, Cortez DV, de Castro HF (2019) Keggin-structure heteropolyacid supported on alumina to be used in trans/esterification of high-acid feedstocks. RSC Adv 9(41):23450–23458. https://doi.org/10.1039/C9RA04300D
Carvalho AKF, Bento HBS, Izário Filho HJ, de Castro HF (2018) Approaches to convert Mucor circinelloides lipid into biodiesel by enzymatic synthesis assisted by microwave irradiations. Renew Energy 125:747–754. https://doi.org/10.1016/j.renene.2018.03.012
Paiva EJM, da Silva MLCP, Barboza JCS, de Oliveira PC, de Castro HF, Giordani DS (2013) Non-edible babassu oil as a new source for energy production–a feasibility transesterification survey assisted by ultrasound. Ultrason Sonochem 20(3):833–838. https://doi.org/10.1016/j.ultsonch.2012.11.003
Talebi AF, Tabatabaei M, Chisti Y (2014) BiodieselAnalyzer: a user-friendly software for predicting the properties of prospective biodiesel. Biofuel Res J 1(2):55–57. https://doi.org/10.18331/BRJ2015.1.2.4
Huang G, Chen F, Wei D, Zhang X, Chen G (2010) Biodiesel production by microalgal biotechnology. Appl Energy 87(1):38–46. https://doi.org/10.1016/j.apenergy.2009.06.016
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process 48(6):1146–1151. https://doi.org/10.1016/j.cep.2009.03.006
Pereira FM, Loures CCA, Amaral MS, Gomes FM, Pedro GA, Machado MAG, Reis CER, Silva MB (2018) Evaluation of fatty acids production by Chlorella minutissima in batch bubble-column photobioreactor. Fuel 230:155–162. https://doi.org/10.1016/j.fuel.2018.04.170
Peng L, Zhang Z, Cheng P, Wang Z, Lan CQ (2016) Cultivation of Neochloris oleoabundans in bubble column photobioreactor with or without localized deoxygenation. Bioresour Technol 206:255–263. https://doi.org/10.1016/j.biortech.2016.01.081
Francisco EC, Neves DB, Jacob-Lopes E, Franco TT (2010) Microalgae as feedstock for biodiesel production: carbon dioxide sequestration, lipid production and biofuel quality. J Chem Technol Biotechnol 85(3):395–403. https://doi.org/10.1002/jctb.2338
Pegallapati AK, Nirmalakhandan N (2013) Internally illuminated photobioreactor for algal cultivation under carbon dioxide-supplementation: performance evaluation. Renew Energy 56:129–135. https://doi.org/10.1016/j.renene.2012.09.052
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31(7):1043–1049. https://doi.org/10.1007/s10529-009-9975-7
Trentacoste EM, Shrestha RP, Smith SR, Glé C, Hartmann AC, Hildebrand M, Gerwick WH (2013) Metabolic engineering of lipid catabolism increases microalgal lipid accumulation without compromising growth. Proc Natl Acad Sci 110(49):19748–19753. https://doi.org/10.1073/pnas.1309299110
Pruvost J, Van Vooren G, Le Gouic B, Couzinet-Mossion A, Legrand J (2011) Systematic investigation of biomass and lipid productivity by microalgae in photobioreactors for biodiesel application. Bioresour Technol 102(1):150–158. https://doi.org/10.1016/j.biortech.2010.06.153
Ho S-H, Chen C-Y, Chang J-S (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102(1):100–112. https://doi.org/10.1002/bit.22033
Pratoomyot J, Srivilas P, Noiraksar T (2005) Fatty acids composition of 10 microalgal species. Songklanakarin J Sci Technol 27(6):1179–1187
Amaral MS, Loures CC, Da Rós P, Machado SA, Reis CER, de Castro HF, Silva MB (2015) Evaluation of the cultivation conditions of marine microalgae Chlorella sp. to be used as feedstock in ultrasound-assisted ethanolysis. Biofuel Res J 2(3):288–294. https://doi.org/10.18331/BRJ2015.2.3.7
Talebi AF, Mohtashami SK, Tabatabaei M, Tohidfar M, Bagheri A, Zeinalabedini M, Mirzaei HH, Mirzajanzadeh M, Shafaroudi SM, Bakhtiari S (2013) Fatty acids profiling: a selective criterion for screening microalgae strains for biodiesel production. Algal Res 2(3):258–267. https://doi.org/10.1016/j.algal.2013.04.003
Ma X-N, Chen T-P, Yang B, Liu J, Chen F (2016) Lipid production from Nannochloropsis. Mar Drugs 14(4):61. https://doi.org/10.3390/md14040061
Hulatt CJ, Wijffels RH, Bolla S, Kiron V (2017) Production of fatty acids and protein by Nannochloropsis in flat-plate photobioreactors. PLoS One 12(1):e0170440. https://doi.org/10.1371/journal.pone.0170440
Patil V, Källqvist T, Olsen E, Vogt G, Gislerød HR (2007) Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac Int 15(1):1–9. https://doi.org/10.1007/s10499-006-9060-3
Tsuzuki M, Ohnuma E, Sato N, Takaku T, Kawaguchi A (1990) Effects of CO2 concentration during growth on fatty acid composition in microalgae. Plant Physiol 93(3):851–856. https://doi.org/10.1104/pp.93.3.851
Cuellar-Bermudez SP, Romero-Ogawa MA, Vannela R, Lai YS, Rittmann BE, Parra-Saldivar R (2015) Effects of light intensity and carbon dioxide on lipids and fatty acids produced by Synechocystis sp. PCC6803 during continuous flow. Algal Res 12:10–16. https://doi.org/10.1016/j.algal.2015.07.018
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86(10):1059–1070. https://doi.org/10.1016/j.fuproc.2004.11.002
Lapuerta M, Rodríguez-Fernández J, Armas O (2010) Correlation for the estimation of the density of fatty acid esters fuels and its implications. A proposed biodiesel cetane index. Chem Phys Lipids 163(7):720–727. https://doi.org/10.1016/j.chemphyslip.2010.06.004
Galadima A, Muraza O (2014) Biodiesel production from algae by using heterogeneous catalysts: a critical review. Energy 78:72–83. https://doi.org/10.1016/j.energy.2014.06.018
Alsalme A, Kozhevnikova EF, Kozhevnikov IV (2008) Heteropoly acids as catalysts for liquid-phase esterification and transesterification. Appl Catal A Gen 349(1–2):170–176. https://doi.org/10.1016/j.apcata.2008.07.027
Ma G, Hu W, Pei H, Jiang L, Ji Y, Mu R (2015) Study of KOH/Al2O3 as heterogeneous catalyst for biodiesel production via in situ transesterification from microalgae. Environ Technol 36(5):622–627. https://doi.org/10.1080/09593330.2014.954629
Ma G, Hu W, Pei H, Jiang L, Song M, Mu R (2015) In situ heterogeneous transesterification of microalgae using combined ultrasound and microwave irradiation. Energy Convers Manag 90:41–46. https://doi.org/10.1016/j.enconman.2014.10.061
Guldhe A, Singh B, Rawat I, Bux F (2014) Synthesis of biodiesel from Scenedesmus sp. by microwave and ultrasound assisted in situ transesterification using tungstated zirconia as a solid acid catalyst. Chem Eng Res Des 92(8):1503–1511. https://doi.org/10.1016/j.cherd.2014.05.012
Knothe G, Matheaus AC, Ryan Iii TW (2003) Cetane numbers of branched and straight-chain fatty esters determined in an ignition quality tester. Fuel 82(8):971–975. https://doi.org/10.1016/S0016-2361(02)00382-4
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16(1):143–169. https://doi.org/10.1016/j.rser.2011.07.143
Funding
Authors are thankful to FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for Grants #16/10636-8, #17/12908-8, and #18/01386-3; to CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brazil)—Finance Code 001; and to Conselho Nacional de Desenvolvimento Científico e Tecnológico CNPq (Process Number 433248/2018-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zorn, S.M.F.E., Reis, C.E.R., Bento, H.B.S. et al. In Situ Transesterification of Marine Microalgae Biomass via Heterogeneous Acid Catalysis. Bioenerg. Res. 13, 1260–1268 (2020). https://doi.org/10.1007/s12155-020-10151-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10151-6