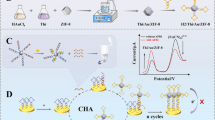
Abstract
A fluorescence method for the quantitative detection of chloramphenicol (CAP) has been developed using phosphate and fluorescent dye 6-carboxy-x-rhodamine (ROX) double-labeled aptamers of CAP and the bimetallic organic framework nanomaterial Cu/UiO-66. Cu/UiO-66 was prepared by coordinate bonding of metal organic framework (MOF) nanomaterial UiO-66 with copper ions. Cu/UiO-66 contains a large number of metal defect sites, which can be combined with phosphate-modified nucleic acid aptamers through strong coordination between phosphate and zirconium to form “fluorescence turn-on” sensors. In the absence of CAP, all single-stranded aptamers were adsorbed on the surface of Cu/UiO-66 through π-π stacking between single-stranded DNA and Cu/UiO-66, which brings the ROX fluorophores and Cu/UiO-66 into close proximity. The ROX fluorescence of aptamers was then quenched by Cu/UiO-66 through photoinduced electron transfer (PET). In the presence of CAP, however, CAP reacted with nucleic acid aptamers to form a special spatial structure, in which the ROX fluorophores were far away from the MOF surface via a change in the spatial structure of the aptamers, and the fluorescence of ROX was able to be recovered. The quantitative detection of CAP can be achieved by measuring the fluorescence signal of ROX using synchronous scanning fluorescence spectrometry. Under optimum conditions, the fluorescence intensities of ROX exhibit a good linear dependence on the concentration of CAP in the range of 0.2–10 nmol/L, with a detection limit of 0.09 nmol/L. The method has advantages of high sensitivity, good selectivity, and a low limit of detection.

Graphical abstract








Similar content being viewed by others
References
Hussein MMA, Wada S, Hatai K, Yamamoto A. Antimycotic activity of eugenol against selected water molds. J Aquat Anim Health. 2000;12(3):224–9. https://doi.org/10.1577/1548-8667(2000)012<0224:aaoeas>2.0.co;2.
Vanderzwalmen M, Eaton L, Mullen C, Henriquez F, Carey P, Snellgrove D, et al. The use of feed and water additives for live fish transport. Rev Aquac. 2019;11(1):263–78. https://doi.org/10.1111/raq.12239.
Bartlett JG. Chloramphenicol. Med Clin N Am. 1982;66(1):91–102. https://doi.org/10.1016/s0025-7125(16)31444-4.
Lu XW, Dang Z, Yang C. Preliminary investigation of chloramphenicol in fish, water and sediment from freshwater aquaculture pond. Int J Environ Sci Technol. 2009;6(4):597–604. https://doi.org/10.1007/bf03326100.
Eom KS. Antibiotic-induced increase in inflammatory markers in cured infectious spondylitis : two case reports. J Korean Neurosurg Soc. 2019;62(4):487–91. https://doi.org/10.3340/jkns.2018.0052.
Couce A, Blazquez J. Side effects of antibiotics on genetic variability. FEMS Microbiol Rev. 2009;33(3):531–8. https://doi.org/10.1111/j.1574-6976.2009.00165.x.
Thomas VM, Thomas-Eapen N. An uncommon side effect of a commonly used antibiotic: amoxicillin-clavulanic acid induced hepatitis. Korean J Fam Med. 2017;38(5):307–10. https://doi.org/10.4082/kjfm.2017.38.5.307.
Cunha BA. Antibiotic side effects. Med Clin N Am. 2001;85(1):149–85. https://doi.org/10.1016/s0025-7125(05)70309-6.
Vaudaux P, Lew DP. Tolerance of staphylococci to bactericidal antibiotics. Injury-Int J Care Inj. 2006;37:15–9. https://doi.org/10.1016/j.injury.2006.04.004.
Moudgil P, Bedi JS, Aulakh RS, Gill JPS, Kumar A. Validation of HPLC multi-residue method for determination of fluoroquinolones, tetracycline, Sulphonamides and chloramphenicol residues in bovine Milk. Food Anal Meth. 2019;12(2):338–46. https://doi.org/10.1007/s12161-018-1365-0.
Nagata T, Oka H. Detection of residual chloramphenicol, florfenicol, and thiamphenicol in yellowtail fish muscles by capillary gas chromatography mass spectrometry. J Agric Food Chem. 1996;44(5):1280–4. https://doi.org/10.1021/jf950343u.
Xu H, Zhang J, He J, Mi JB, Liu LS. Rapid detection of chloramphenicol in animal products without clean-up using LC-high resolution mass spectrometry. Food Addit Contam Part A-Chem. 2011;28(10):1364–71. https://doi.org/10.1080/19440049.2011.598466.
Du XJ, Zhou XN, Li P, Sheng W, Ducancel F, Wang S. Development of an immunoassay for chloramphenicol based on the preparation of a specific single-chain variable fragment antibody. J Agric Food Chem. 2016;64(14):2971–9. https://doi.org/10.1021/acs.jafc.6b00639.
El-Moghazy AY, Zhao CY, Istamboulie G, Amaly N, Si Y, Noguer T, et al. Ultrasensitive label-free electrochemical immunosensor based on PVA-co-PE nanofibrous membrane for the detection of chloramphenicol residues in milk. Biosens Bioelectron. 2018;117:838–44. https://doi.org/10.1016/j.bios.2018.07.025.
Zhou XC, Shi J, Zhang J, Zhao K, Deng AP, Li JG. Multiple signal amplification chemiluminescence immunoassay for chloramphenicol using functionalized SiO2 nanoparticles as probes and resin beads as carriers. Spectroc Acta Pt A-Molec Biomolec Spectr. 2019;222:117177. https://doi.org/10.1016/j.saa.2019.117177.
Xu Q, Song ZJ, Ji ST, Xu G, Shi WY, Shen LX. The photocatalytic degradation of chloramphenicol with electrospun Bi2O2CO3-poly(ethylene oxide) nanofibers: the synthesis of crosslinked polymer, degradation kinetics, mechanism and cytotoxicity. RSC Adv. 2019;9(51):29917–26. https://doi.org/10.1039/c9ra06346c.
Cardoso AR, Marques AC, Santos L, Carvalho AF, Costa FM, Martins R, et al. Molecularly-imprinted chloramphenicol sensor with laser-induced graphene electrodes. Biosens Bioelectron. 2019;124:167–75. https://doi.org/10.1016/j.bios.2018.10.015.
Sun YF, Wei TT, Jiang MD, Xu LH, Xu ZX. Voltammetric sensor for chloramphenicol determination based on a dual signal enhancement strategy with ordered mesoporous carbon@polydopamine and beta-cyclodextrin. Sens Actuator B-Chem. 2018;255:2155–62. https://doi.org/10.1016/j.snb.2017.09.016.
He X, Wu C, Qian Y, Li Y, Zhang L, Ding F, et al. Highly sensitive and selective light-up fluorescent probe for monitoring gallium and chromium ions in vitro and in vivo. Analyst. 2019;144(12):3807–16. https://doi.org/10.1039/C9AN00625G.
He X, Xiong W, Zhang L, Xu C, Fan J, Qian Y, et al. ESIPT-based ratiometric fluorescent probe for highly selective and sensitive sensing and bioimaging of group IIIA ions in living cancer cells and zebrafish. Dyes Pigment. 2020;174:108059. https://doi.org/10.1016/j.dyepig.2019.108059.
Sharma R, Akshath US, Bhatt P, Raghavarao K. Fluorescent aptaswitch for chloramphenicol detection - quantification enabled by immobilization of aptamer. Sens Actuator B-Chem. 2019;290:110–7. https://doi.org/10.1016/j.snb.2019.03.093.
Yaghi OM, O'Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Reticular synthesis and the design of new materials. Nature. 2003;423(6941):705–14. https://doi.org/10.1038/nature01650.
Geng DT, Zhang M, Hang XX, Xie WJ, Qin YC, Li Q, et al. A 2D metal-thiacalix 4 arene porous coordination polymer with 1D channels: gas absorption/separation and frequency response. Dalton Trans. 2018;47(27):9008–13. https://doi.org/10.1039/c8dt02089b.
Jiang YS, Song JS, Xiu ZJ, Huang LL, Gao F, Jiao SF, et al. Solvent-free syntheses of two pcu topological indium phosphite-oxalates with a novel butterfly motif and proton conductivity. Micropor Mesopor Mat. 2019;289:109643. https://doi.org/10.1016/j.micromeso.2019.109643.
Zhang M, Chen MW, Bi YF, Huang LL, Zhou K, Zheng ZP. A bimetallic Co4Mo8 cluster built from Mo-8 oxothiomolybdate capped by a co-4-thiacalix 4 arene unit: the observation of the co-Mo synergistic effect for binder-free electrocatalysts. J Mater Chem A. 2019;7(20):12893–9. https://doi.org/10.1039/c9ta01603a.
Song YF, Cronin L. Postsynthetic covalent modification of metal–organic framework (MOF) materials. Angew Chem Int Ed. 2008;47(25):4635–7. https://doi.org/10.1002/anie.200801631.
Yassine O, Shekhah O, Assen AH, Belmabkhout Y, Salama KN, Eddaoudi M. H2S sensors: fumarate-based fcu-MOF thin film grown on a capacitive interdigitated electrode. Angew Chem Int Ed. 2016;55(51):15879–83. https://doi.org/10.1002/anie.201608780.
Han Y-H, Tian C-B, Li Q-H, Du S-W. Highly chemical and thermally stable luminescent EuxTb1−x MOF materials for broad-range pH and temperature sensors. J Mater Chem C. 2014;2(38):8065–70. https://doi.org/10.1039/C4TC01336K.
Saraf M, Rajak R, Mobin SM. A fascinating multitasking cu-MOF/rGO hybrid for high performance supercapacitors and highly sensitive and selective electrochemical nitrite sensors. J Mater Chem A. 2016;4(42):16432–45. https://doi.org/10.1039/C6TA06470A.
Smith LM, Sanders JZ, Kaiser RJ, Hughes P, Dodd C, Connell CR, et al. Fluorescence detection in automated dna-sequence analysis. Nature. 1986;321(6071):674–9. https://doi.org/10.1038/321674a0.
Liu WS, Qu XY, Zhu CF, Gao YH, Mao CJ, Song JM, et al. A two-dimensional zinc(II)-based metal-organic framework for fluorometric determination of ascorbic acid, chloramphenicol and ceftriaxone. Microchim Acta. 2020;187:136. https://doi.org/10.1007/s00604-019-3979-3.
Wang Q, Du XM, Zhao B, Pang ML, Li Y, Ruan WJ. A luminescent MOF as a fluorescent sensor for the sequential detection of Al3+ and phenylpyruvic acid. New J Chem. 2020;44(4):1307–12. https://doi.org/10.1039/c9nj05439a.
Yu L, Zheng QT, Wang H, Liu CX, Huang XQ, Xiao YX. Double-color lanthanide metal-organic framework based logic device and visual Ratiometric fluorescence water microsensor for solid pharmaceuticals. Anal Chem. 2020;92(1):1402–8. https://doi.org/10.1021/acs.analchem.9b04575.
Wang ZH, Wang XZ, Lai XY, Hou Q, Ma JX, Li JH, et al. Three new coordination polymers based on a fluorene derivative ligand for the highly luminescent sensitive detection of Fe3+. J Mol Struct. 2020;1202:127341. https://doi.org/10.1016/j.molstruc.2019.127341.
Zhang YJ, Zhao DS, Liu ZJ, Yang JD, Niu XY, Fan LM, et al. Synthesis of two isostructural Zn-CPs and their fluorescence sensing for Cr (VI) ion and nitrofurantoin in aqueous medium. J Solid State Chem. 2020;282:121086. https://doi.org/10.1016/j.jssc.2019.121086.
Liu QJ, Wang H, Han P, Feng XY. Fluorescent aptasensing of chlorpyrifos based on the assembly of cationic conjugated polymer-aggregated gold nanoparticles and luminescent metal-organic frameworks. Analyst. 2019;144(20):6025–32. https://doi.org/10.1039/c9an00943d.
Sun C, Zhao SY, Qu F, Han WL, You JM. Determination of adenosine triphosphate based on the use of fluorescent terbium(III) organic frameworks and aptamer modified gold nanoparticles. Microchim Acta. 2020;187:34. https://doi.org/10.1007/s00604-019-4019-z.
Wang HS, Liu HL, Wang K, Ding Y, Xu JJ, Xia XH, et al. Insight into the unique fluorescence quenching property of metal-organic frameworks upon DNA binding. Anal Chem. 2017;89(21):11366–71. https://doi.org/10.1021/acs.analchem.7b02256.
Ye T, Liu YF, Luo M, Xiang X, Ji XH, Zhou GH, et al. Metal-organic framework-based molecular beacons for multiplexed DNA detection by synchronous fluorescence analysis. Analyst. 2014;139(7):1721–5. https://doi.org/10.1039/c3an02077k.
Li NN, Huang X, Sun DP, Yu WY, Tan WG, Luo ZF, et al. Dual-aptamer-based voltammetric biosensor for the mycobacterium tuberculosis antigen MPT64 by using a gold electrode modified with a peroxidase loaded composite consisting of gold nanoparticles and a Zr(IV)/terephthalate metal-organic framework. Microchim Acta. 2018;185:543. https://doi.org/10.1007/s00604-018-3081-2.
Guo JF, Li CM, Hu XL, Huang CZ, Li YF. Metal-organic framework MIL-101 enhanced fluorescence anisotropy for sensitive detection of DNA. RSC Adv. 2014;4(18):9379–82. https://doi.org/10.1039/c3ra47389a.
Deria P, Bury W, Hod I, Kung C-W, Karagiaridi O, Hupp JT, et al. MOF functionalization via solvent-assisted ligand incorporation: phosphonates vs carboxylates. Inorg Chem. 2015;54(5):2185–92. https://doi.org/10.1021/ic502639v.
X-m H, Li N, Wang K, Zhang Z-q, Zhang J, Dang F-q. A fluorescence aptasensor based on two-dimensional sheet metal-organic frameworks for monitoring adenosine triphosphate. Anal Chim Acta. 2018;998:60–6. https://doi.org/10.1016/j.aca.2017.10.028.
Liu Y, Hou W, Xia L, Cui C, Wan S, Jiang Y, et al. ZrMOF nanoparticles as quenchers to conjugate DNA aptamers for target-induced bioimaging and photodynamic therapy. Chem Sci. 2018;9(38):7505–9. https://doi.org/10.1039/C8SC02210K.
Wang H-S, Liu H-L, Wang K, Ding Y, Xu J-J, Xia X-H, et al. Insight into the unique fluorescence quenching property of metal-organic frameworks upon DNA binding. Anal Chem. 2017;89(21):11366–71. https://doi.org/10.1021/acs.analchem.7b02256.
Javidi M, Housaindokht MR, Verdian A, Razavizadeh BM. Detection of chloramphenicol using a novel apta-sensing platform based on aptamer terminal-lock in milk samples. Anal Chim Acta. 2018;1039:116–23. https://doi.org/10.1016/j.aca.2018.07.041.
Abdel-Mageed AM, Rungtaweevoranit B, Parlinska-Wojtan M, Pei X, Yaghi OM, Behm RJ. Highly active and stable single-atom cu catalysts supported by a metal–organic framework. J Am Chem Soc. 2019;141(13):5201–10. https://doi.org/10.1021/jacs.8b11386.
Yan C, Zhang J, Yao L, Xue F, Lu J, Li B, et al. Aptamer-mediated colorimetric method for rapid and sensitive detection of chloramphenicol in food. Food Chem. 2018;260:208–12. https://doi.org/10.1016/j.foodchem.2018.04.014.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (21465010), the Open Foundation of Key Laboratory of Biologic Resources Protection and Utilization of Hubei Province (PKLHB1918, PKLHB1730), Hubei Provincial Natural Science Foundation of China (2018CFB247), and Special Funds for “Double First-Class” Construction in Hubei Province.
Funding
National Natural Science Foundation of China (21465010),
Open Foundation of Key Laboratory of Biologic Resources Protection and Utilization of Hubei Province (PKLHB1918, PKLHB1730),
Hubei Provincial Natural Science Foundation of China (2018CFB247).
Special Funds for “Double First-Class” Construction in Hubei Province.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animal interest
This research did not involve human participants and/or animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 806 kb)
Rights and permissions
About this article
Cite this article
Lu, Z., Jiang, Y., Wang, P. et al. Bimetallic organic framework-based aptamer sensors: a new platform for fluorescence detection of chloramphenicol. Anal Bioanal Chem 412, 5273–5281 (2020). https://doi.org/10.1007/s00216-020-02737-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02737-y