Article contents
Effect of anisotropic silk fibroin topographies on dorsal root ganglion
Published online by Cambridge University Press: 03 June 2020
Abstract
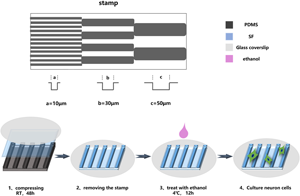
The surface topology of biomaterial has a definite effect on the growth behavior of nerve cells for peripheral nerve regeneration. In this study, the silk fibroin (SF) film with different anisotropic microgroove/ridge was constructed by micropatterning technology. The effects of topologies width on the directional growth of dorsal root ganglion (DRG) neurons were evaluated. The results showed that the topological structure of the SF film with higher SF concentration was more clear and complete. The microtopography of the SF film with a concentration of 15% and a groove width of around 30 μm could effectively guide the directional growth of the nerve fibers of DRG. And nerve fibers could obviously form nerve fiber bundles which may have a certain pavement effect on the recovery of nerve function. The study indicated that the SF film with a specific width of the topological structure may have potential applications in the field of directional nerve regeneration.
- Type
- Article
- Information
- Journal of Materials Research , Volume 35 , Issue 13: Focus Section: Interactions of Shear Transformation Bands , 14 July 2020 , pp. 1738 - 1748
- Copyright
- Copyright © Materials Research Society 2020
References
- 7
- Cited by





