Community Preferences of Woody Plant Species in a Heterogeneous Temperate Forest, China
- 1College of Life Sciences, Henan Agricultural University, Zhengzhou, China
- 2College of Forestry, Henan Agricultural University, Zhengzhou, China
Diversified community types provide different microhabitats for plant growth. However, whether the distribution of species is random distribution or ecological specialization within different plant community types remains to be elucidated. Here, five 1 ha (100 m × 100 m) plots with different communities were established in a temperate forest. We examined community structure differences by non-metric multidimensional scaling and betadisper test, analyzed the species–community relationships by correlation network approach, and then the examined distribution preferences of woody plant species by torus-translation test. Results showed that the abundance, richness, and species composition of woody plants exhibited significant differences among the five communities. The specialization index showed that 42.83% of the species had the characteristics of distribution specialization for different communities. The torus-translation test showed that 85 species (86.74%) were positively associated with specific community. Our findings suggested that the distribution of woody plants species among different plant community types is not random but specialization. Different woody plant species have distinct specific preferences among various plant community types in temperate mountain forest. These findings provide new insights into the biodiversity conservation of woody plant species in temperate deciduous broad-leaved forests and indicate the potential importance of community partitioning for the maintenance of woody plant diversity.
Introduction
The diversity maintenance mechanism of woody plants is becoming increasingly clear (Zhu et al., 2019). Niche theory states that species coexistence can be attributed to species differences in terms of environmental requirements and the distribution of environmental conditions in space (Silvertown, 2004; Chen et al., 2018). If niche-relevant environmental conditions are spatially structured, then they should be reflected in species distributions through the associations of species with different habitats (Svenning, 1999; Gao et al., 2017). Consequently, distinct species assemblages should be formed at the community level (Svenning, 1999; Gao et al., 2017). Many woody plants in forest ecosystems have distinct specific microhabitat preferences and have highlighted the importance of microhabitat characteristics in defining species assemblages (Svenning, 1999; Harms et al., 2001; Comita et al., 2007; Lai et al., 2009). However, these observations highlight the importance of topographic habitat factors (Svenning, 1999; Harms et al., 2001; Comita et al., 2007; Lai et al., 2009), whereas the role of community partitioning in the maintenance of woody plant diversity remains poorly known.
Different communities with various dominant species exhibit several differences in community structure, understory vegetation, light availability, soil nutrient, etc. (Jia et al., 2019). Thus, these communities also provide different microhabitats for the growth of plants, animals, and microorganisms (Yuan et al., 2012). Compared with dominant species, less attention has been paid to non-dominant species in the community (Yuan et al., 2012). Most common species have stable reproduction and good environmental adaptability (Bevill and Louda, 1999). Some occasional or rare species have harsh environmental conditions for their growth (Barlow et al., 2010). Whether the distribution of species is random distribution or ecological specialization within different plant community types remains to be elucidated.
Temperate mountain forests are widely distributed in northern China and harbor a high diversity of communities (Hou, 1983). These communities have wide variations in species composition and diversity, which presumably provide niches to accommodate diverse woody plant. However, human interference and environmental damage decrease the number and diversity of plant community in temperate mountain forests, with some species even at risk of extinction. Elucidating the role of community types in the maintenance of woody plant diversity is a crucial step to conserve their diversity. We hope that this study can provide insights into the distribution characteristics of woody plant species within different plant community types, thereby providing a basis for assessing its role in diversity maintenance of temperate mountain forest ecosystems.
As stated above, we have posed the question of whether the distribution of species is random distribution or ecological specialization within different plant community types. To test this hypothesis, we established five 1 ha (100 m × 100 m) plots with different communities in temperate forest. We examined community structure differences by using the Kruskal–Wallis method, non-metric multidimensional scaling (NMDS), and betadisper test. Then, we analyzed the species–community relationships among different forest communities by correlation network approach. Lastly, we examined the distribution preferences of woody plant species by torus-translation test. We hope that this study can provide an opportunity to improve forest biodiversity conservation.
Materials and Methods
Study Site and Sampling
This study was conducted in a temperate deciduous broad-leaved forest in the Baotianman National Nature Reserve, East China (111°47′–112°04′ E, 33°20′–33°36′ N, c. 53.4 km2, 1600–1845 m above sea level). The highest peak, at an elevation of 1830 m, gives an indication of the complexity of the areas’ varied topography and climate, which has been named as a “World Biosphere Reserve.” The average annual temperature is 15.1°C, the average annual precipitation is 855.6 mm, the annual evaporation is 991.6 mm, and the average annual relative humidity is 68% (Jia et al., 2019).
The forest coverage in the reserve is 97.3%. The dominant broad-leaved tree species in the reserve are mainly Quercus aliena var. acutiserrata, Quercus variabilis, and Quercus glandulifera var. brevipetiolata. The dominant needle-leaved tree species in is mainly Pinus armandii. The shrub layer is mainly composed of Forsythia suspensa, Philadelphus incanus, and Lindera obtusiloba. The herbaceous layer is mainly composed of Lysimachia fortunei and Aconitum hemsleyanum (Jia et al., 2019).
Five 1 ha (100 m × 100 m) plots with different community types were established in the survey area on the basis of the comprehensive investigation of the Baotianman National Nature Reserve. Each plot was divided into 100 quadrates (10 m × 10 m). All stems ≥1 cm diameters at breast height in the Baiyunshan plot were tagged, mapped, and measured (Condit, 1995; Supplementary Table S1). Elevation, convex concave, slope, and aspect were calculated for each 10 m × 10 m quadrate in the plot (Supplementary Table S2) in accordance with the study of Harms et al. (2001).
Data Analysis
On the basis of the important value (IV), the dominant species in the plots were statistically analyzed. The IV was calculated as follows: IV = (relative abundance (%) + relative frequency (%) + relative breast height sectional area (%)/3. The species accumulation curves of woody plants in five communities were drawn using the “specaccum” function in the vegan package of R.
The Kruskal–Wallis method was used to test for differences in the four topographic factors of the five communities. Redundancy analysis (RDA) was used to detect species–environment relationships. In RDA, the species matrix is the species abundant in plots. The environmental matrix includes elevation, convex concave, slope, and aspect. Monte Carlo permutation test on the basis of 999 permutations was used to analyze whether the model reached a significant level (P < 0.05). The “envfit” function in the R language software with vegan package was used to test the significance of each environmental factor and species distribution (Oksanen et al., 2007).
The Kruskal–Wallis method was used to test for differences in the richness and abundance of woody plants in the five communities. The species composition of woody plants was analyzed by ordination using NMDS with the Bray–Curtis dissimilarity, and community types were fitted as centroids onto the NMDS graph using the envfit function. Permutational multivariate ANOVA (PERMANOVA) was applied to explore the significant differences on the basis of 999 permutations. Non-metric multidimensional scaling was conducted using the metaMDS command in the vegan package of R (Oksanen et al., 2007). Afterward, we assessed the effect of community partitioning on the beta diversity of woody plants by running the “betadisper” function. ANOVA was performed to test whether these distances differed among community types. Betadisper test was conducted using the “betadisper” command in the vegan package of R (Oksanen et al., 2007).
A correlation network approach was used to visualize potential relationships between woody plants and community types. We evaluated the structure of the plant–community network using the H2′ metric of specialization and connectance index (Blüthgen et al., 2007). The architecture of the plant–community network was visualized using the “bipartite” package of R (Dormann et al., 2009).
Associations of woody plant species with community types were determined using torus-translation tests considering the spatial autocorrelation of species distribution (Harms et al., 2001; Lai et al., 2009). The fundamental of the torus-translation test is to randomly calculate species distribution probability under a null model. In our study, on the basis of the 10 m × 10 m subplot in five 1 ha plots with different community types, the method provides 1 real and 1999 translated maps. If the real relative density of species in the focal community was greater or smaller than at least 97.5% of the simulated relative densities, then it was determined to be statistically positively or negatively associated with that community (α = 0.05 level of significance for a two-tailed test). Community associations were only tested for species with more than five individuals in the 5 ha plot. Nearly 61.25% of the species (98/160) recorded greater than five individuals in the 5 ha plot. Further details on this method are provided by Harms et al. (2001). Torus-translation test analyses were conducted in R3.4.0.
Results
Species Composition Among the Five Communities
A total of 160 species of woody plants in the five communities were recorded (Supplementary Table S1). Seventy-nine species were observed in the Q. variabilis community, and Q. variabilis was the dominant species (IV = 21.21). Seventy-six species were observed in the Q. glandulifera var. brevipetiolata community, and Q. glandulifera var. brevipetiolata was the dominant species (IV = 23.38). The species diversity in mixed broadleaf–conifer forest was the lowest in the five communities, with 47 species of woody plants dominated by Q. aliena var. acutiserrata (IV = 29.72) and P. armandii (IV = 17.90). The species diversity in Q. aliena var. acutiserrata forest was the highest in the five communities, with 95 species of woody plants dominated by Q. aliena var. acutiserrata (IV = 34.97). Eighty-three species were observed in the miscellaneous wood forest community, and no evident dominant species was observed in this community (Table 1).
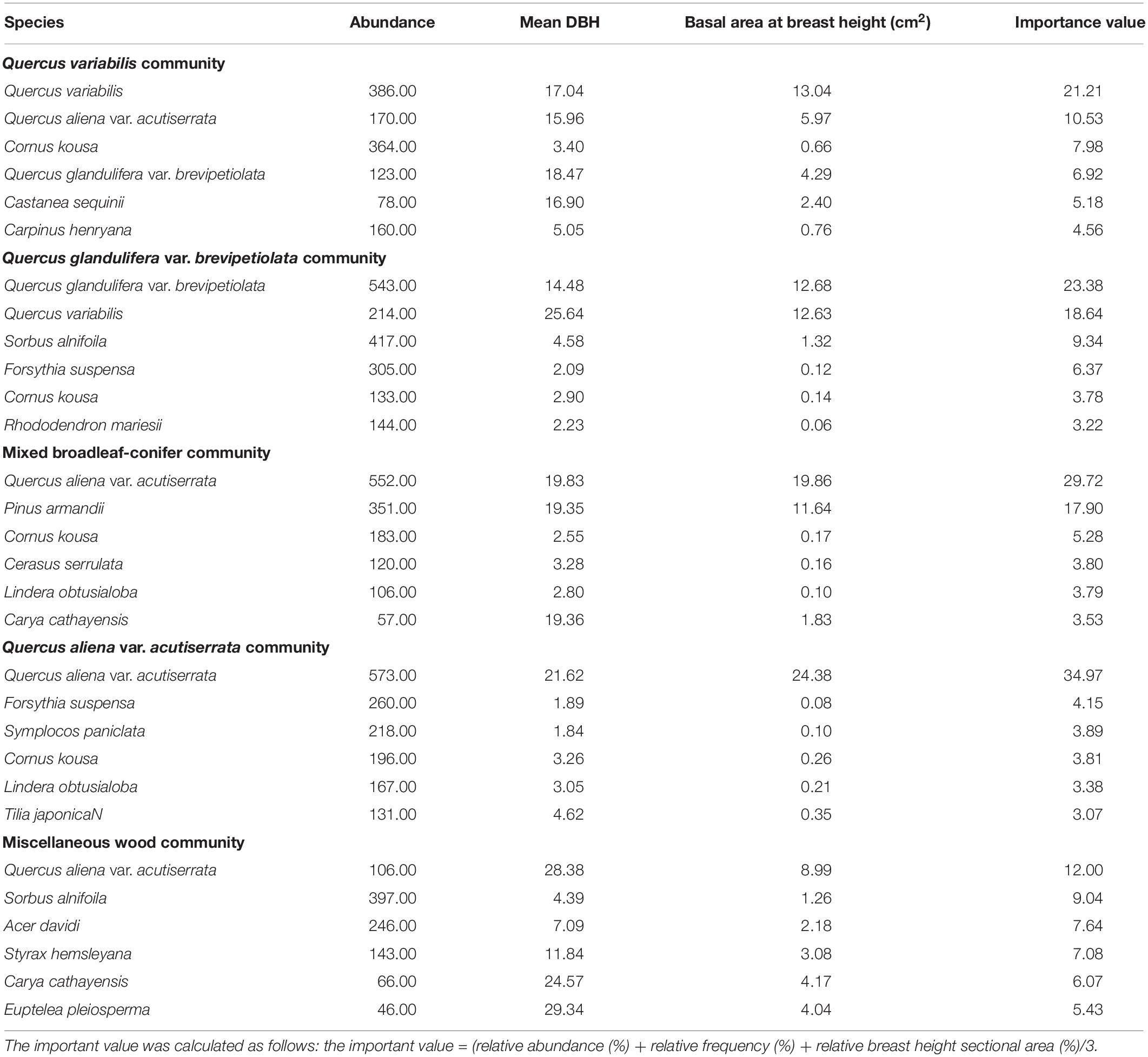
The spatial distribution of species in the five communities is shown in Figure 1. The species density of the Q. aliena var. acutiserrata community was the highest (2912 individuals), followed by the Q. glandulifera var. brevipetiolata community (2340 individuals). The numbers of individuals in the Q. variabilis, mixed broadleaf–conifer, and miscellaneous wood communities were 2197, 2049, and 2156, respectively. Species accumulation curves also showed different species richness in the five communities (Figure 2).
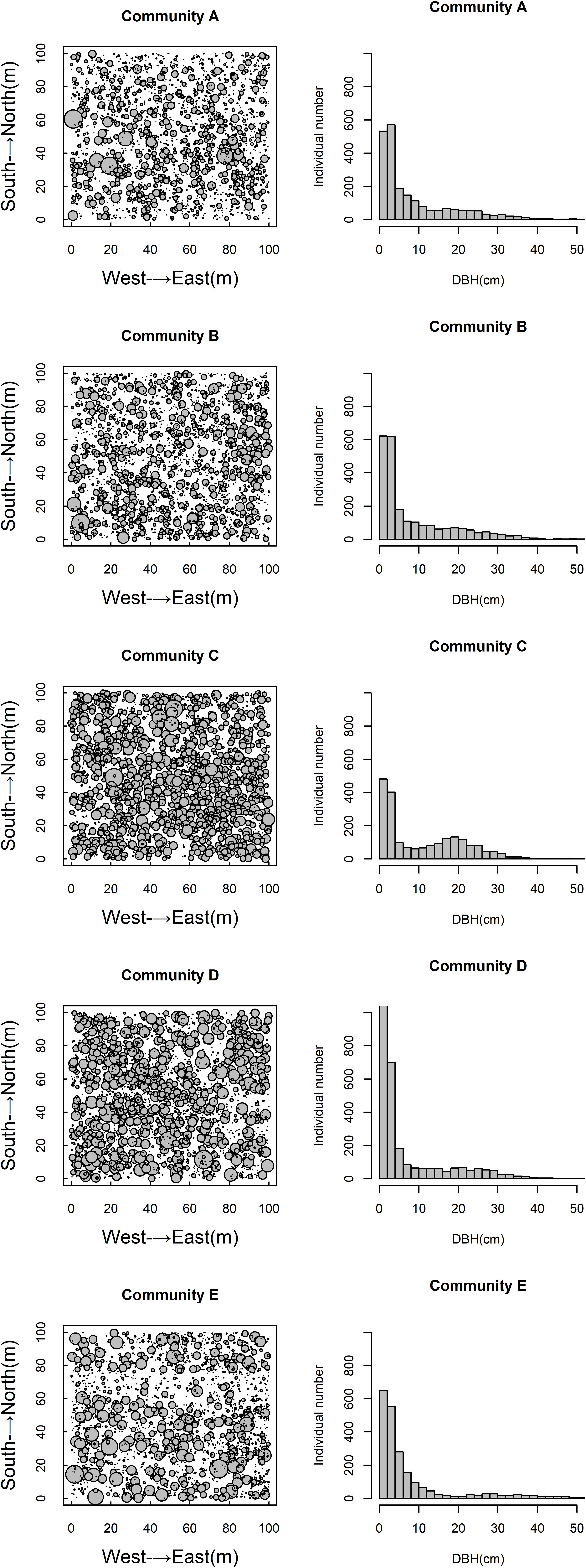
Figure 1. Spatial scatter plots and DBH plots of woody plants in different communities. A, B, C, D, and E were the Quercus variabilis community, Quercus glandulifera var. brevipetiolata community, Mixed broadleaf-conifer community, Quercus aliena var. acutiserrata community, and Miscellaneous wood community, respectively.
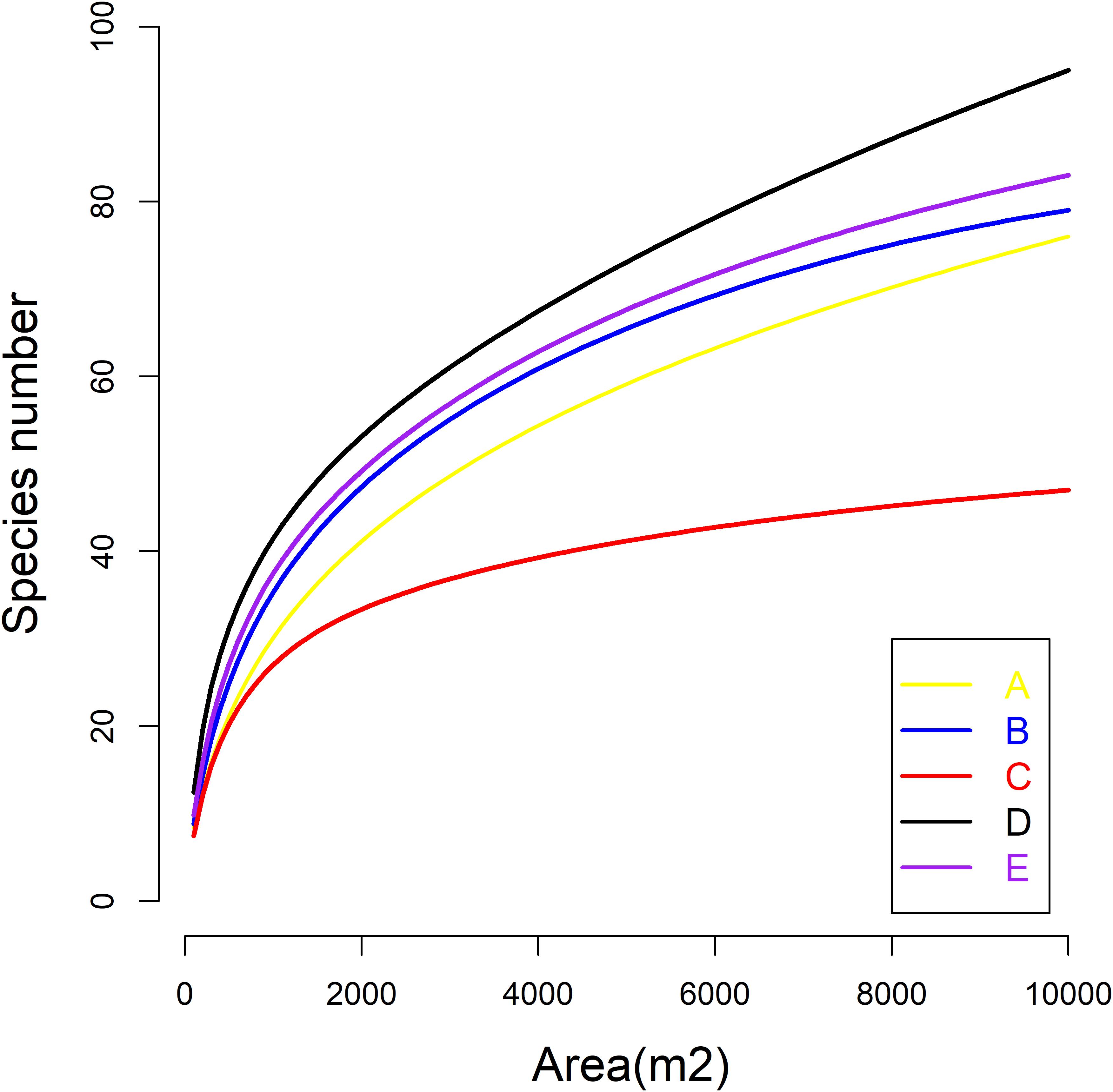
Figure 2. Species accumulation curves of five communities. A, B, C, D, and E were the Quercus variabilis community, Quercus glandulifera var. brevipetiolata community, Mixed broadleaf-conifer community, Quercus aliena var. acutiserrata community, and Miscellaneous wood community, respectively.
The Kruskal–Wallis test showed that the differences in elevation, convex concave, slope, and aspect among the five communities were significant (Supplementary Figure S1). In RDA, topographic variables explained 7.16% of the variation in the species distribution (F = 9.5444, P < 0.001). Elevation (r2 = 0.429, P < 0.001), aspect (r2 = 0.159, P < 0.001), and convex concave (r2 = 0.021, P < 0.005) were the most important factors (Supplementary Figure S2).
The Kruskal–Wallis test showed that the differences in species abundance (A) and species richness (B) among the five communities were significant (Figure 3). Non-metric multidimensional scaling followed by PERMANOVA also showed distinct species compositions among the five communities (F = 29.061, R2 = 49.2, P = 0.001) (Figure 4). Betadisper analysis followed by ANOVA showed significant differences in the community dispersion of woody plants in the five communities (F = 10.775, P = 0.001) (Figure 5).
Figure 3. Species richness (A) and abundance (B) of woody plants in five communities. Black lines indicate significant differences, as obtained using the Kruskal–Wallis method (P ≤ 0.05 level of significance). A, B, C, D, and E were the Quercus variabilis community, Quercus glandulifera var. brevipetiolata community, Mixed broadleaf-conifer community, Quercus aliena var. acutiserrata community, and Miscellaneous wood community, respectively.
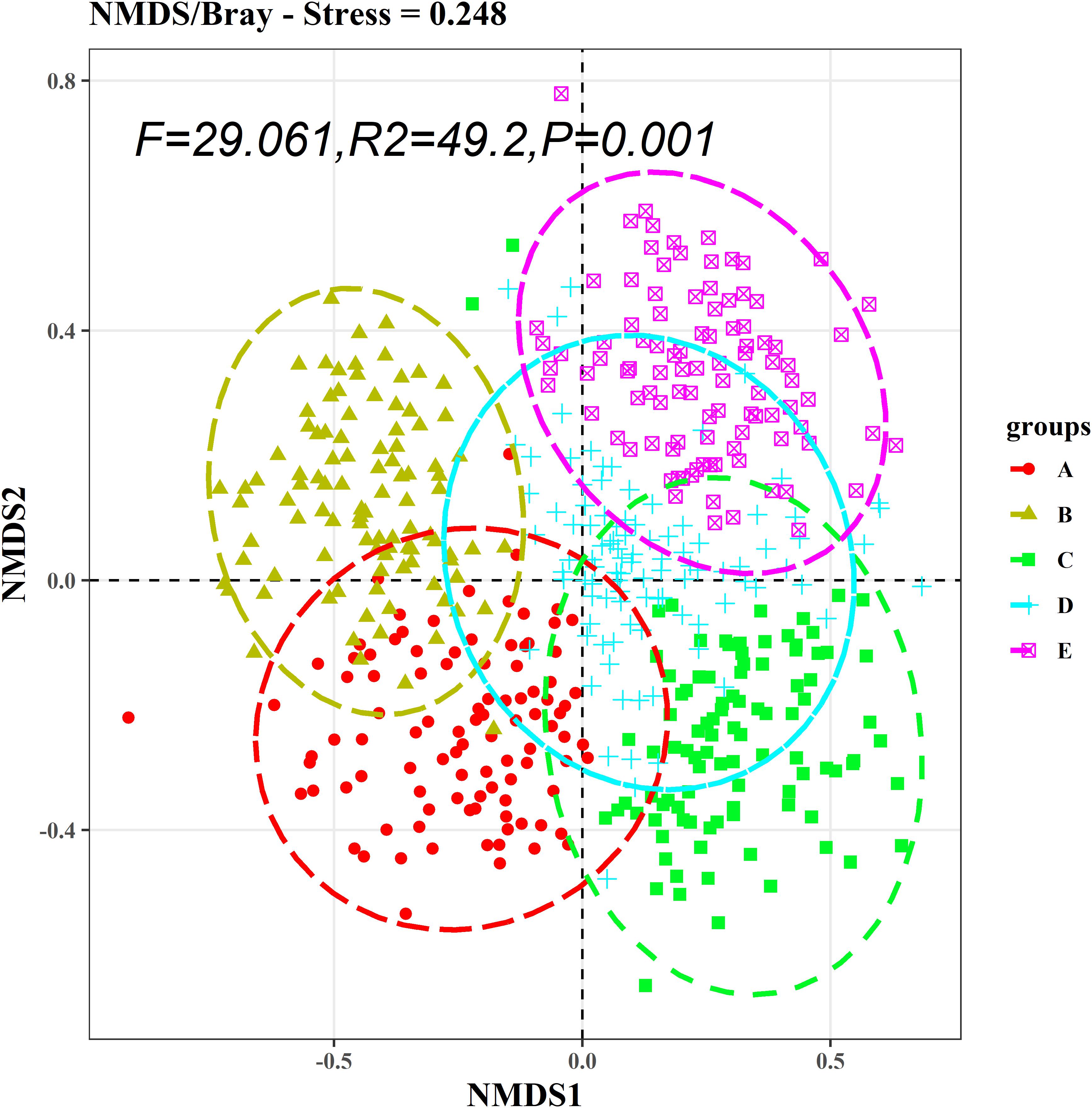
Figure 4. NMDS analysis of species composition among five communities. PERMANOVA was used to evaluate intercommunity significance. A, B, C, D, and E were the Quercus variabilis community, Quercus glandulifera var. brevipetiolata community, Mixed broadleaf-conifer community, Quercus aliena var. acutiserrata community, and Miscellaneous wood community, respectively.
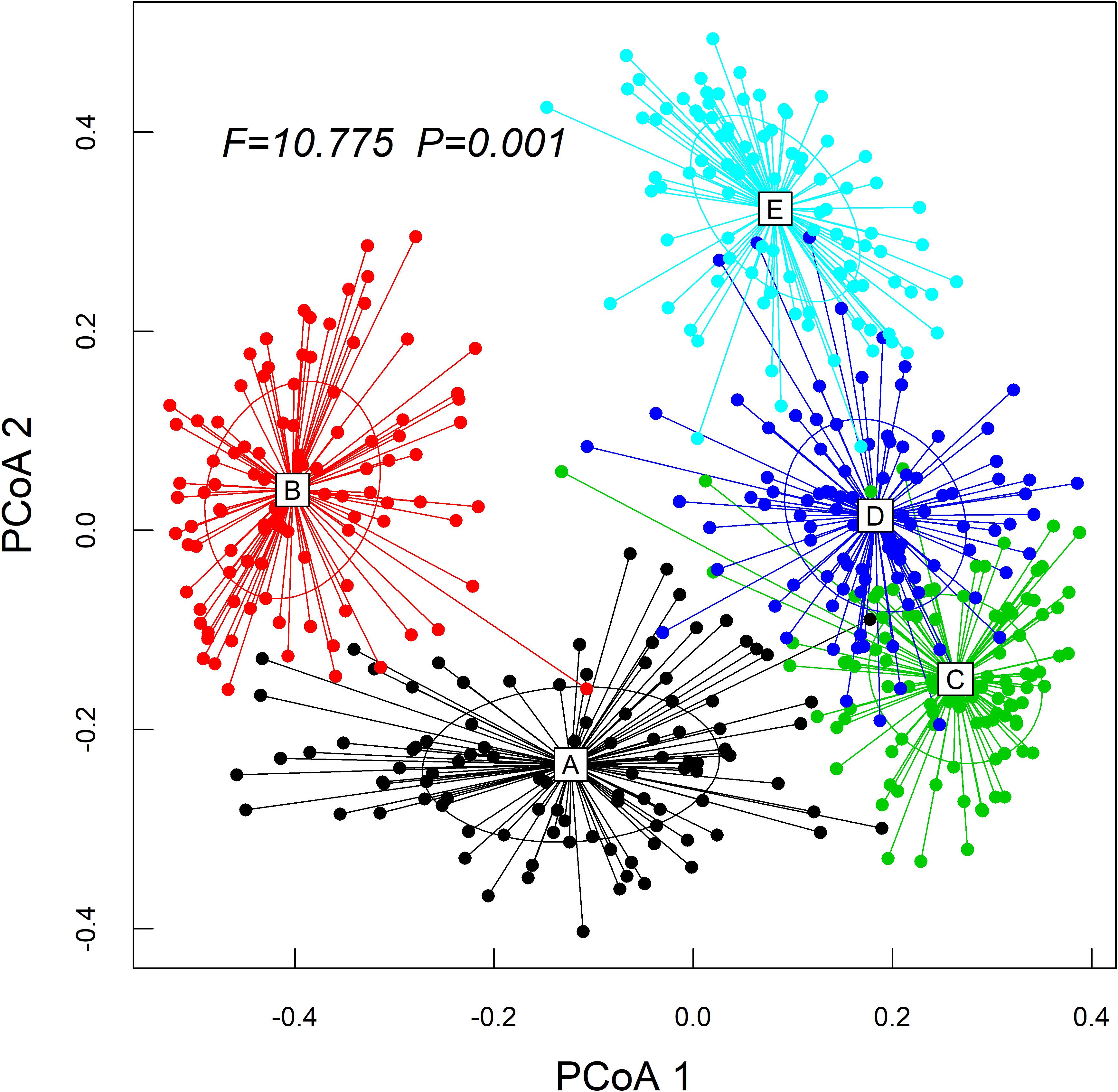
Figure 5. Effect of community types on beta diversity of the woody plant by running the betadisper function. ANOVA was applied to test how these distances differed among the communities. PCoA1 and PCoA2 are the first and second sort axes in the “betadisper” analysis, respectively.
Specialization Characteristics of Species Distribution
A total of 10, 10, 4, 16, and 17 species were recorded in the Q. variabilis, Q. glandulifera var. brevipetiolata, mixed broadleaf–conifer, Q. aliena var. acutiserrata, and miscellaneous wood communities, respectively. There were 13 species were shared by the five communities (Figure 6). In network analysis, the linkage index showed that 46.91% of the potential associations between species and communities were detected. The specialization index showed that 42.83% of the species had the characteristics of distribution specialization for different communities (Figure 7).
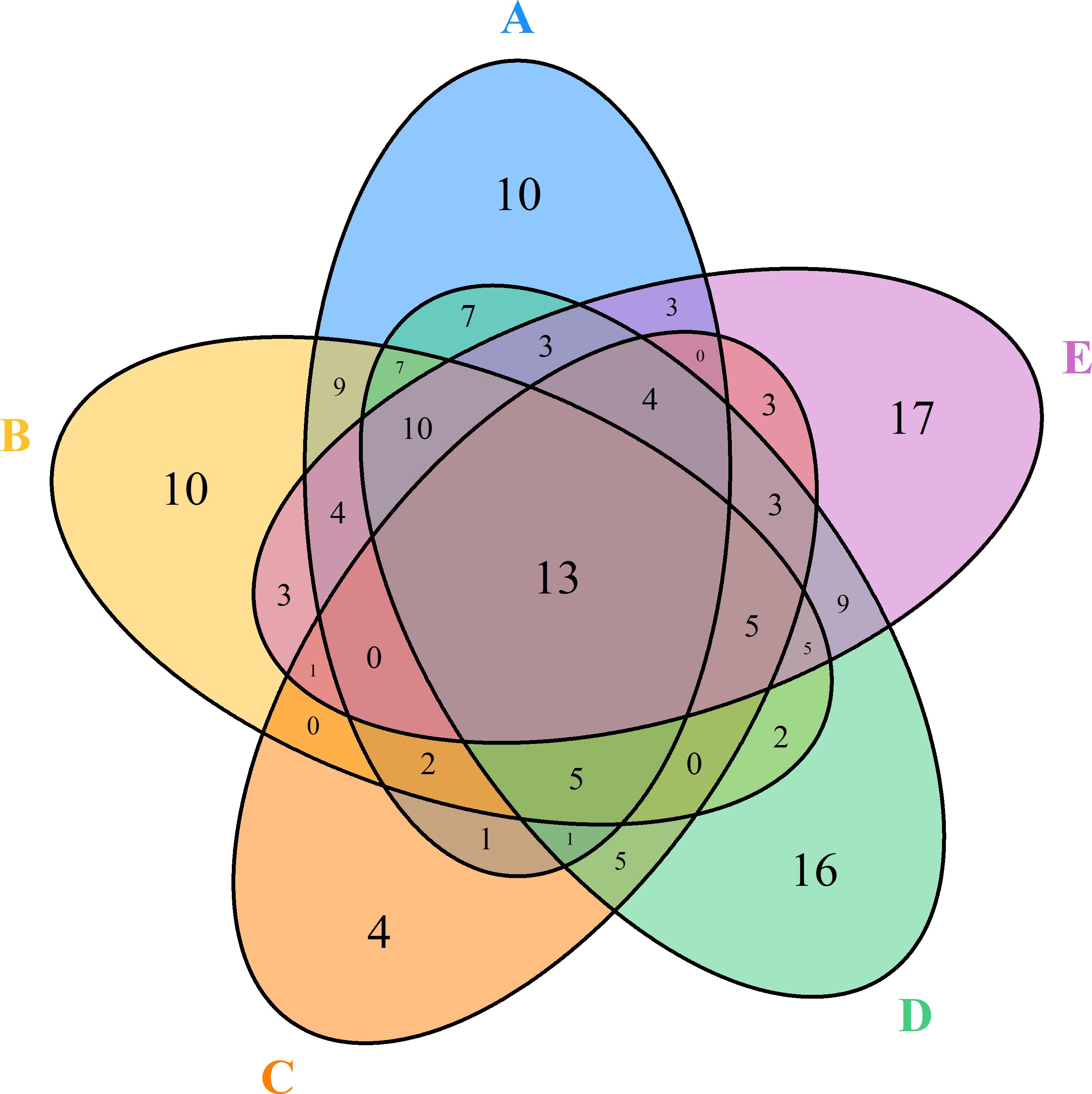
Figure 6. Degree of overlap of the species composition in the five communities. A, B, C, D, and E were the Quercus variabilis community, Quercus glandulifera var. brevipetiolata community, Mixed broadleaf-conifer community, Quercus aliena var. acutiserrata community, and Miscellaneous wood community, respectively.
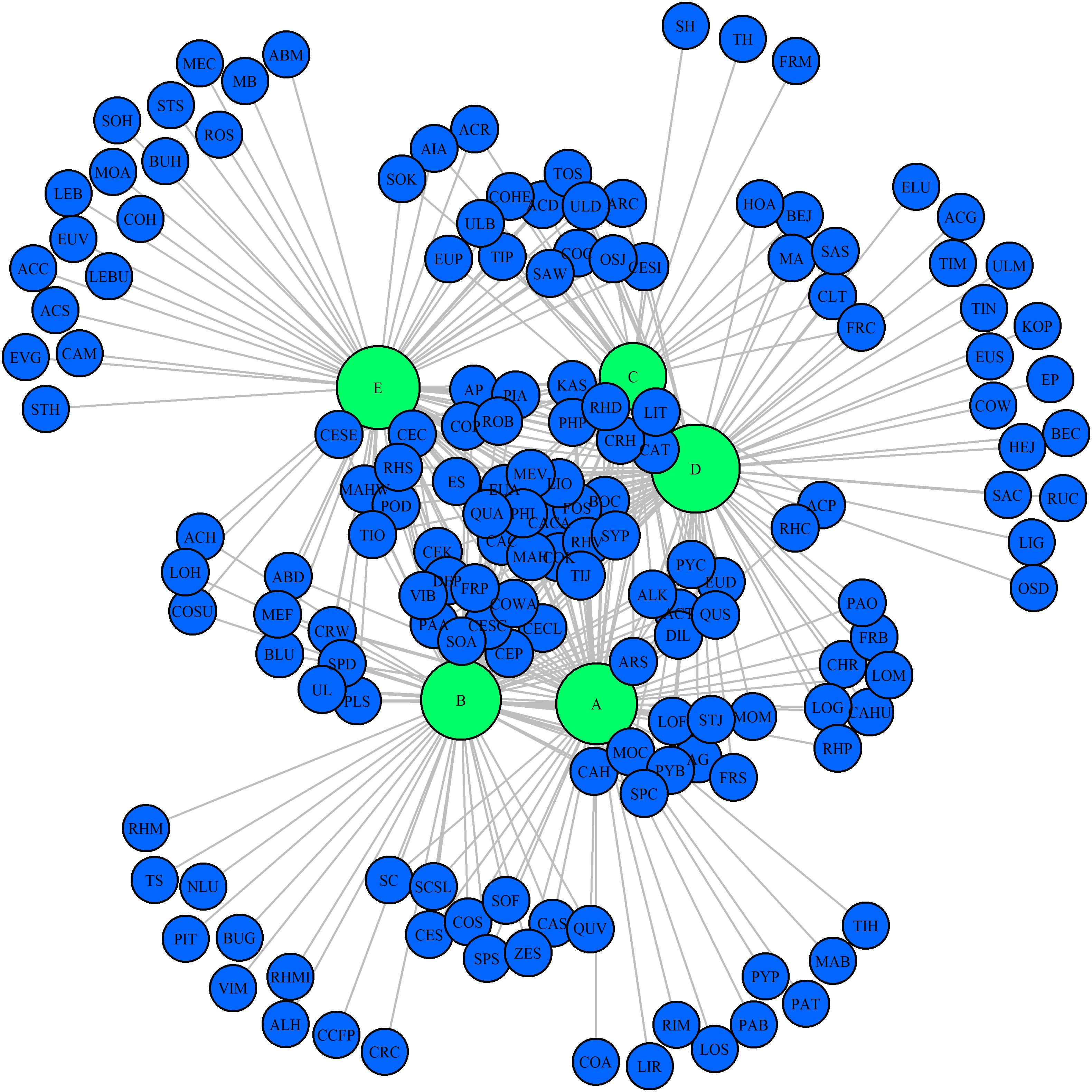
Figure 7. Network analysis of species composition among the five communities. The size of the dot indicates the abundance of species. A, B, C, D, and E were the Quercus variabilis community, Quercus glandulifera var. brevipetiolata community, Mixed broadleaf-conifer community, Quercus aliena var. acutiserrata community, and Miscellaneous wood community, respectively. The abbreviations of species are shown in Supplementary Table S3.
Associations Between Species and Community Types
On the basis of the torus-translation tests, 85 species that were examined were associated with the community (85/98, 86.74%). A total of 17, 15, 19, 16, and 18 species showed positive associations (P < 0.05) to Q. variabilis community, Q. glandulifera var. brevipetiolata community, Mixed broadleaf-conifer community, Q. aliena var. acutiserrata community, and Miscellaneous wood community, respectively. Thirteen of the species examined were not associated with community. No species were negatively associated with the community. The detailed associations of the species with communities are shown in Table 2.
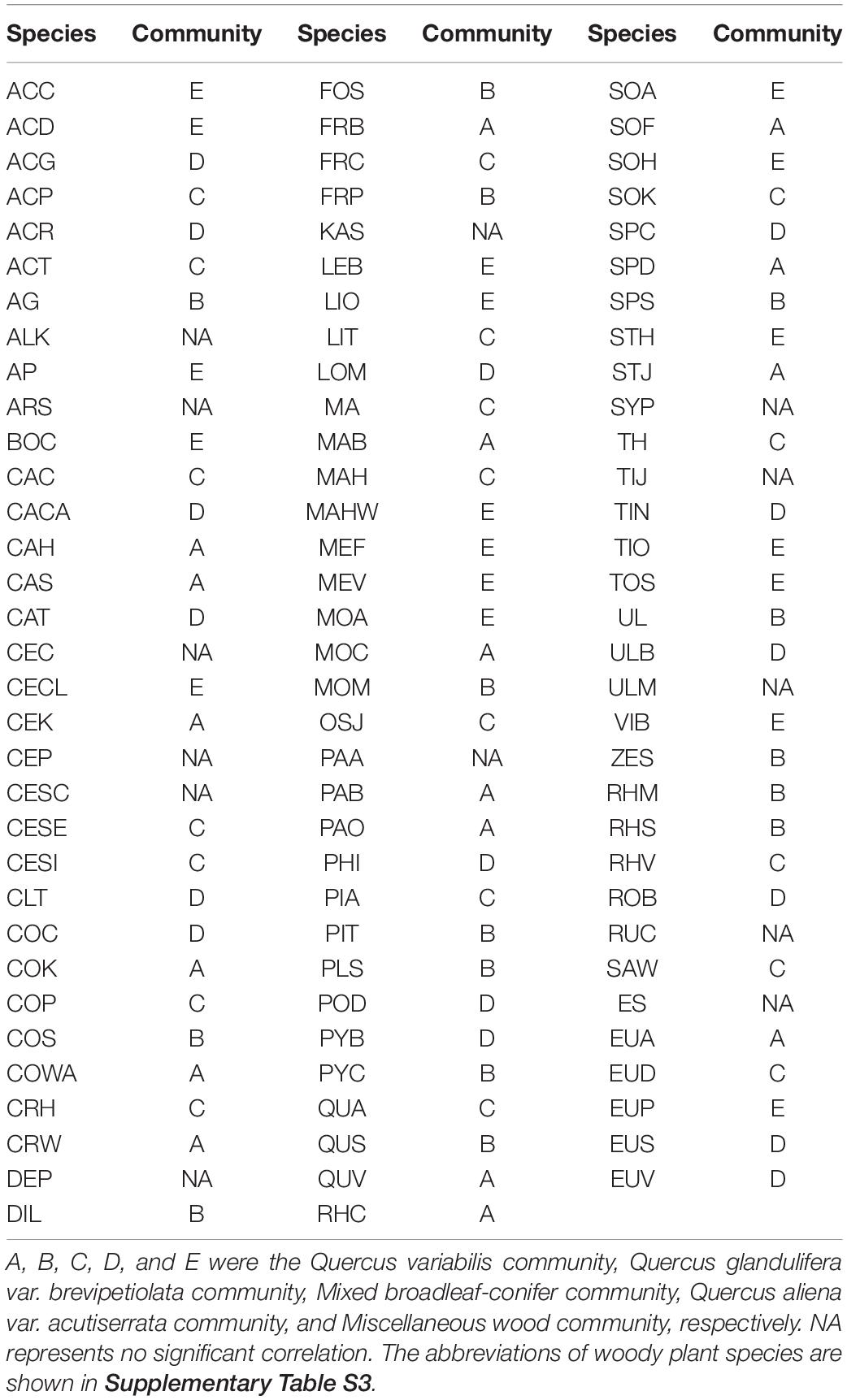
Table 2. Significant associations of woody plant species with five communities (P ≤ 0.05 level of significance for torus-translation test).
Discussion
Forest ecosystems consist of biological and abiotic factors. In our study, topography, as an important abiotic factor, accounted only 7.16% variations in the distribution of species. Topography played a small role in the distribution of species in the five communities. Plant community is an important biological factor and plays an important role in forest ecosystems (Jia et al., 2019). In the present study, network analysis showed that specialization was 0.428, which was higher than those of the previously reported plant–fungus (0.265) (Toju et al., 2014) and plant–seed diffusion networks (0.354) (Dicks et al., 2002) and slightly lower than that of the plant–pollination network (0.533) (Bartomeus et al., 2008). This result indicated that the distribution pattern of most woody plant species among different plant community types was not random but specialization.
The torus-translation test showed that 85 species (86.74%) were positively associated with a specific community. In terms of the biological factors, the differences in the abundance, richness, and species compositions of the woody plants among the five communities were significant. In terms of the abiotic environment, different plant communities can reshape soil physical and chemical properties, humidity, litter composition, and light availability (Niu et al., 2016; Jia et al., 2019). Topography also played a small role in the distribution of species in the five communities. Therefore, the environmental conditions within the community were significantly different among various types of plant communities. This phenomenon may be an important reason why woody plant species exhibited ecological preferences for different community types. However, the ecological preferences of species were largely due to different community types. Topography only played a small role in the ecological preferences of the species. Given the lack of soil data, light data, and so on, the role of community type in determining species distribution preference had not been directly quantified.
Many studies on the spatial distribution and drivers of woody plants are available. In particular, the effects of abiotic factors, such as topography (Svenning, 1999; Wangda and Ohsawa, 2006; Lan et al., 2011), light (Han et al., 2017; Gallé et al., 2018), and soil (Song et al., 2015; Gao et al., 2017), and biological factors, such as competition (Zhu et al., 2015, 2018) and species succession (Mi et al., 2016; Sun et al., 2017), on woody plants have been investigated. In the present study, different woody plant species had distinct specific preferences among various plant community types in the temperate mountain forest.
The torus-translation test also showed that only 13 species that were examined were not significantly associated with any community. These species were mainly shrubs, and no evident ecological preference may be related to their resistance to stress. Meanwhile, no species were negatively associated with the community. The result agreed with our expectation. Given that the forest communities in this study were nearly 100 years old, species that were not suitable for this area may have been eliminated early. Therefore, all species could survive here, and most species (86.74%) could find a very suitable microhabitat in this region.
Conclusion
The distribution of woody plant species among different plant community types was not random but specialization. Different woody plant species had distinct specific preferences among various plant community types in the temperate mountain forest. These findings suggested that community partitioning is important for woody plant distribution and potentially important for the maintenance of woody plant diversity.
In terms of sustainable forest management, diverse woody plant communities should be maintained on the basis of our results because the diversity of communities can promote the increase in diversity for woody plants. Our results also indicated that different woody plants may have distinct specific community preferences. Therefore, different protection strategies should be used in different woody plant species on the basis of their distinct community preferences.
Only the distribution characteristics of woody plant species among community types were studied. The specific contributions of biological (e.g., plant community, microorganism community), abiotic (e.g., soil, humidity, and light), and spatial factors (e.g., dispersal limitation) to woody plant space should be quantified in future studies. Our study only found the ecological preference of woody species for community type by means of field survey and statistical test. The relationship between the physiological characteristics of woody species and their distribution preference will be the focus of our future studies.
Data Availability Statement
All datasets generated for this study are included in the article/Supplementary Material.
Author Contributions
TW originally formulated the idea. YC and YS developed methodology, performed statistical analyses, and wrote the manuscript. JX, ZY, and YY conducted fieldwork.
Funding
YC considers this work a contribution to his Young Talents project funded by Henan Agricultural University (111/30500744), and Youth talent lift project funded by Henan Province (2020HYTP037). ZY considers this work a contribution to his NSFC project funded by China (U1704115), and the key scientific research projects of higher school of Henan Province (No. 18A180011). TW considers this work a contribution to his National Natural Science Foundation of China (31270493), and the Mountain-river-forest- farmland- lake-grassland Ecological Restoration Pre-Project of Henan Province (Jiyuan) (No. JGZJ-CAI-2019125).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the Forestry Bureau in Luoyang and Wumasi forestry farm in Songxian for their support for the plot construction and species survey.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fevo.2020.00165/full#supplementary-material
References
Barlow, J., Gardner, T. A., Louzada, J., and Peres, C. A. (2010). Measuring the conservation value of tropical primary forests: the effect of occasional species on estimates of biodiversity uniqueness. PLoS One 5:e9609. doi: 10.1371/journal.pone.0009609
Bartomeus, I., Vilà, M., and Santamaría, L. (2008). Contrasting effects of invasive plants in plant–pollinator networks. Oecologia 155, 761–770. doi: 10.1007/s00442-007-0946-1
Bevill, R. L., and Louda, S. M. (1999). Comparisons of related rare and common species in the study of plant rarity. Conserv. Biol. 13, 493–498. doi: 10.1046/j.1523-1739.1999.97369.x
Blüthgen, N., Menzel, F., Hovestadt, T., Fiala, B., and Blüthgen, N. (2007). Specialization, constraints, and conflicting interests in mutualistic networks. Curr. Biol. 17, 341–346. doi: 10.1016/j.cub.2006.12.039
Chen, Y., Yuan, Z., Bi, S., Wang, X., Ye, Y., and Svenning, J. C. (2018). Macrofungal species distributions depend on habitat partitioning of topography, light, and vegetation in a temperate mountain forest. Sci. Rep. 8:13589. doi: 10.1038/s41598-018-31795-7
Comita, L. S., Condit, R., and Hubbell, S. P. (2007). Developmental changes in habitat associations of tropical trees. J. Ecol. 95, 482–492. doi: 10.3732/ajb.1400109
Condit, R. (1995). Research in large, long-term tropical forest plots. Trends Ecol. Evol. 10, 18–22. doi: 10.1016/s0169-5347(00)88955-7
Dicks, L. V., Corbet, S. A., and Pywell, R. F. (2002). Compartmentalization in plant–insect flower visitor webs. J. Anim. Ecol. 71, 32–43. doi: 10.1046/j.0021-8790.2001.00572.x
Dormann, C. F., Fründ, J., Blüthgen, N., and Gruber, B. (2009). Indices, graphs and null models: analyzing bipartite ecological networks. Open Ecol. J. 2, 7–24. doi: 10.2174/1874213000902010007
Gallé, Á, Czékus, Z., Bela, K., Horváth, E., Ördög, A., Csiszár, J., et al. (2018). Plant glutathione transferases and light. Front. Plant Sci. 9:1944.
Gao, C., Shi, N. N., Chen, L., Ji, N. N., Wu, B. W., Wang, Y. L., et al. (2017). Relationships between soil fungal and woody plant assemblages differ between ridge and valley habitats in a subtropical mountain forest. New Phytol. 213, 1874–1885. doi: 10.1111/nph.14287
Han, B., Umaña, M. N., Mi, X., Liu, X., Chen, L., Wang, Y., et al. (2017). The role of transcriptomes linked with responses to light environment on seedling mortality in a subtropical forest. China. J. Ecol. 105, 592–601. doi: 10.1111/1365-2745.12760
Harms, K. E., Condit, R., Hubbell, S. P., and Foster, R. B. (2001). Habitat associations of trees and shrubs in a 50-ha Neotropical forest plot. J. Ecol. 89, 947–959. doi: 10.1111/j.1365-2745.2001.00615.x
Hou, H. Y. (1983). Vegetation of China with reference to its geographical distribution. Ann. Miss. Bot. Garden 70, 509–549.
Jia, H. R., Chen, Y., Wang, X. Y., Li, P. K., Yuan, Z. L., and Ye, Y. Z. (2019). The Relationships among Topographically-driven Habitats, Dominant Species and Vertical Layers in Temperate Forest in China. Russian J. Ecol. 50, 172–186. doi: 10.1134/s1067413619020061
Lai, J., Mi, X., Ren, H., and Ma, K. (2009). Species-habitat associations change in a subtropical forest of China. J. Veg. Sci. 20, 415–423. doi: 10.1111/j.1654-1103.2009.01065.x
Lan, G., Hu, Y., Cao, M., and Zhu, H. (2011). Topography related spatial distribution of dominant tree species in a tropical seasonal rain forest in China. For. Ecol. Manag. 262, 1507–1513. doi: 10.1016/j.foreco.2011.06.052
Mi, X., Swenson, N. G., Jia, Q., Rao, M., Feng, G., Ren, H., et al. (2016). Stochastic assembly in a subtropical forest chronosequence: evidence from contrasting changes of species, phylogenetic and functional dissimilarity over succession. Sci. Rep. 6:32596. doi: 10.1038/srep32596
Niu, F., Duan, D., Chen, J., Xiong, P., Zhang, H., Wang, Z., et al. (2016). Eco-physiological responses of dominant species to watering in a natural grassland community on the semi-arid Loess Plateau of China. Front. Plant Sci. 7:663. doi: 10.3389/fpls.2016.00663
Oksanen, J., Kindt, R., Legendre, P., O’Hara, B., Stevens, M. H. H., Oksanen, M. J., et al. (2007). Vegan: Community Ecology Package. Available online at: https://cran.r-project.org/web/packages/vegan/index.html (accessed September 1, 2019).
Silvertown, J. (2004). Plant coexistence and the niche. Trends Ecol. Evol. 19, 605–611. doi: 10.1016/j.tree.2004.09.003
Song, P., Ren, H., Jia, Q., Guo, J., Zhang, N., and Ma, K. (2015). Effects of historical logging on soil microbial communities in a subtropical forest in southern China. Plant Soil 397, 115–126. doi: 10.1007/s11104-015-2553-y
Sun, C., Chai, Z., Liu, G., and Xue, S. (2017). Changes in species diversity patterns and spatial heterogeneity during the secondary succession of grassland vegetation on the Loess Plateau. China. Front. Plant Sci. 8:1465. doi: 10.3389/fpls.2017.01465
Svenning, J. C. (1999). Microhabitat specialization in a species-rich palm community in Amazonian Ecuador. J. Ecol. 87, 55–65. doi: 10.1046/j.1365-2745.1999.00329.x
Toju, H., Guimaraes, P. R., Olesen, J. M., and Thompson, J. N. (2014). Assembly of complex plant–fungus networks. Nat. Commun. 5:5273.
Wangda, P., and Ohsawa, M. (2006). Structure and regeneration dynamics of dominant tree species along altitudinal gradient in a dry valley slopes of the Bhutan Himalaya. For. Ecol. Manag. 230, 136–150. doi: 10.1016/j.foreco.2006.04.027
Yuan, Z., Gazol, A., Wang, X., Xing, D., Lin, F., Bai, X., et al. (2012). What happens below the canopy? Direct and indirect influences of the dominant species on forest vertical layers. Oikos 121, 1145–1153. doi: 10.1111/j.1600-0706.2011.19757.x
Zhu, Y., Comita, L. S., Hubbell, S. P., and Ma, K. (2015). Conspecific and phylogenetic density dependent survival differs across life stages in a tropical forest. J. Ecol. 103, 957–966. doi: 10.1111/1365-2745.12414
Zhu, Y., Liu, Z., and Jin, G. (2019). Evaluating individual-based tree mortality modeling with temporal observation data collected from a large forest plot. For. Ecol. Manag. 450:117496. doi: 10.1016/j.foreco.2019.117496
Keywords: forest community, niche theory, species diversity, dominant species, biodiversity protection
Citation: Chen Y, Shao Y, Xi J, Yuan Z, Ye Y and Wang T (2020) Community Preferences of Woody Plant Species in a Heterogeneous Temperate Forest, China. Front. Ecol. Evol. 8:165. doi: 10.3389/fevo.2020.00165
Received: 22 January 2020; Accepted: 12 May 2020;
Published: 05 June 2020.
Edited by:
Hang Sun, Kunming Institute of Botany, Chinese Academy of Sciences, ChinaReviewed by:
Zhiyao Tang, Peking University, ChinaWanxia Peng, Institute of Subtropical Agriculture, Chinese Academy of Sciences, China
Copyright © 2020 Chen, Shao, Xi, Yuan, Ye and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ting Wang, tingwang126@126.com
†These authors have contributed equally to this work
 Yun Chen
Yun Chen Yizhen Shao
Yizhen Shao Jingjing Xi1
Jingjing Xi1  Zhiliang Yuan
Zhiliang Yuan