Abstract
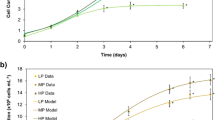
Growing algae in darkness for biodiesel production eliminates the challenges of evaporation and light penetration reported for open ponds and the costs and fouling that plague photobioreactors. The current study demonstrated that Chlorella kessleri str. UTEX 263 could grow heterotrophically in the dark on pure sugars or lignocellulosic hydrolysates of plant biomass. Hydrolysates of a prairie grass native to Kansas, Big Bluestem (Andropogon gerardii), supported the growth of C. kessleri in the dark. Nitrogen limitation stimulated the accumulation of biodiesel lipids by 10-fold in heterotrophic cultures grown on pure sugars or Big Bluestem hydrolysate. Limiting P in the growth medium also was shown to increase cellular lipid accumulation in C. kessleri. Iron limitation was not sufficient to increase cellular lipid content. Crude biomass extracts may have levels of N that cannot be easily removed, which are high enough to relieve N limitations in growth media. This initial study suggests that P might be more easily removed from biomass extracts than N for increasing cellular lipid production by nutrient limitation and further that native prairie grasses are potentially suitable as sources of lignocellulosic sugars.










Similar content being viewed by others
References
Abou-shanab RAI, Jeon BH, Song H, Kim Y, Hwang JH (2006) Algae-biofuel: potential use as sustainable alternative green energy. Online J Power Energ Eng 1:4–6
Adenan NS, Yusoff FM, Medipally SR, Shariff M (2016) Enhancement of lipid production in two marine microalgae under different levels of nitrogen and phosphorus deficiency. J Environ Biol 37:669–676
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol 24:1–15
Barker HA (1935) The metabolism of the colorless alga, Prototheca zopfii Krüger. J Cell Comp Physiol 7:73–93
Behrens PW (2005) Photobioreactors and fermentors: the light and dark side of growing algae. In: Andersen RA (ed) Algal culturing techniques. Academic Press, Burlington, pp 189–204
Belarbi EH, Molina E, Chisti Y (2000) A process for high yield and scaleable recovery of high purity eicosapentaenoic acid esters from microalgae and fish oil. Enzym Microb Technol 26:516–529
Beyerinck MW (1890) Culterversuche mit Zoochlorellen, Lichenengonidien und anderen niederen Algen. I. Das Isoliren niederer Algen durch die Gelatinemethode. Bot Z 47:725–739
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Brennan L, Owende P (2010) Biofuels from microalgae – a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Carvalho AP, Meireles LA, Malcata FX (2008) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Chen F, Johns MR (1991) Effect of C/N ratio and aeration on the fatty acid composition of heterotrophic Chlorella sorokiniana. J Appl Phycol 3:203–209
Chen F, Johns MR (1996) Relationship between substrate inhibition and maintenance energy of Chlamydomonas reinhardtii in heterotrophic culture. J Appl Phycol 8:15–19
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Converti A, Casazza AA, Ortiz EY, Perego P, Del Borghi M (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151
Cooksey KE, Guckert JB, Williams SA, Callis PR (1987) Fluorometric determination of the neutral lipid content of microalgal cells using Nile Red. J Microbiol Methods 6:333–345
Dandinpet KK, Schneegurt MA (2013a) Dark-grown algae fed corn, sorghum, and lignocellulosic hydrolysates for biodiesel production. Trans KS Acad Sci 116:71–72
Dandinpet KK, Schneegurt MA (2013b) Dark-grown algae fed corn and sorghum hydrolysates for biodiesel production. Abstracts and Program, 113th General Meeting of the American Society for Microbiology, Denver, May 2013
Dandinpet KK, Schneegurt MA (2014) Dark-grown algae fed grain and lignocellulosic hydrolysates for biodiesel production. Abstracts and Program, 114th General Meeting of the American Society for Microbiology, Boston, May 2014
Davis R, Aden A, Pienkos PT (2011) Techno-economic analysis of autotrophic microalgae for fuel production. Appl Energy 88:3524–3531
De Swaaf ME, Sijtsma L, Pronk JT (2003) High-cell-density fed-batch cultivation of the docosahexaenoic acid producing marine alga Crypthecodinium cohnii. Biotechnol Bioeng 81:666–672
Demirbas A (2009) Progress and recent trends in biodiesel fuels. Energ Convers Manage 50:14–34
Deng X, Fei X, Li Y (2011) The effects of nutritional restriction on neutral lipid accumulation in Chlamydomonas and Chlorella. Afr J Microbiol Res 5:260–270
Dille JW, Rogers CM, Schneegurt MA (2016) Isolation and characterization of bacteria isolated from the feathers of wild Dark-eyed Juncos (Junco hyemalis). Auk 133:155–167
Dragone G, Fernandes BD, Vicente A, Teixeira JA (2010) Third generation biofuels from microalgae. In: Méndez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex, Badajoz, pp 1355–1366
Endo H, Hosoya H, Koibuchi T (1977) Growth yields of Chlorella regularis in dark-heterotrophic continuous cultures using acetate. J Ferment Technol 55:369–379
Fan J, Cui Y, Wan M, Wang W, Li Y (2014) Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol Biofuels 7:17
García Sánchez JL, Berenguel M, Rodríguez F, Fernández Sevilla JM, Brindley Alias C, Acién Fernández FG (2003) Minimization of carbon losses in pilot-scale outdoor photobioreactors by model-based predictive control. Biotechnol Bioeng 84:533–543
Gigova L, Ivanova NJ (2015) Microalgae respond differently to nitrogen availability during culturing. J Biosci 40:365–374
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Graverholt OS, Eriksen NT (2007) Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Appl Microbiol Biotechnol 77:69–75
Havlík P, Schneider UA, Schmid E, Böttcher H, Fritz S, Skalský R, Aoki K, De Cara S, Kindermann G, Kraxner F, Leduc S, McCallum I, Mosnier A, Sauer T, Obersteiner M (2011) Global land-use implications of first and second generation biofuel targets. Energ Policy 39:5690–5702
Heredia-Arroyo T, Wei W, Hu B (2010) Oil accumulation via heterotrophic/mixotrophic Chlorella protothecoides. Appl Biochem Biotechnol 162:1978–1995
Hill J, Nelson E, Tilman D, Polasky S, Tiffany D (2006) Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc Natl Acad Sci U S A 103:11206–11210
Kay RA, Barton LL (1991) Microalgae as food and supplement. Crit Rev Food Sci Nutr 30:555–573
Kern JD, Hise AM, Characklis GW, Gerlach R, Viamajala S, Gardner RD (2017) Using life cycle assessment and techno-economic analysis in a real options framework to inform the design of algal biofuel production facilities. Bioresour Technol 225:418–428
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Lee RA, Lavoie JM (2013) From first- to third-generation biofuels: challenges of producing a commodity from a biomass of increasing complexity. Anim Front 3:6–11
Li X, Xu H, Wu Q (2007) Large-scale biodiesel production from microalga Chlorella protothecoides through heterotrophic cultivation in bioreactors. Biotechnol Bioeng 98:764–771
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic, and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liang K, Zhang Q, Gu M, Cong W (2013) Effect of phosphorus on lipid accumulation in freshwater microalga Chlorella sp. J Appl Phycol 25:311–318
Lincoln SF (2005) Fossil fuels in the 21st century. Ambio 34:621–627
Liu ZY, Wang GC, Zhou BC (2008) Effect of iron on growth and lipid accumulation in Chlorella vulgaris. Bioresour Technol 99:4717–4722
Louw TM, Griffiths MJ, Jones SMJ, Harrison STL (2016) Techno-economics of algal biodiesel. In: Bux F, Chisti Y (eds) Algae biotechnology. Springer, Cham, pp 111–141
Mandal S, Mallick N (2009) Microalga Scenedesmus obliquus as a potential source for biodiesel production. Appl Microbiol Biotechnol 84:281–291
Miao X, Wu Q (2004) High yield bio-oil production from fast pyrolysis by metabolic controlling of Chlorella protothecoides. J Biotechnol 110:85–93
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Milne TA, Evans RJ, Nagle N (1990) Catalytic conversion of microalgae and vegetable oils to premium gasoline, with shape-selective zeolites. Biomass 21:219–232
Moore A (2008) Biofuels are dead: long live biofuels(?) – part one. New Biotechnol 25:6–12
Mühlroth A, Winge P, El Assimi A, Jouhet J, Maréchal E, Hohmann-Marriott MF, Vadstein O, Bones AM (2017) Mechanisms of phosphorus acquisition and lipid class remodeling under P limitation in a marine microalga. Plant Physiol 175:1543–1559
Mutlu YB, Işik O, Uslu L, Koç K, Durmaz Y (2011) The effects of nitrogen and phosphorus deficiencies and nitrite addition on the lipid content of Chlorella vulgaris (Chlorophyceae). Afr J Biotechnol 10:453–456
Nagarajan D, Lee DJ, Chang JS (2018) Heterotrophic microalgal cultivation. In: Liao Q, Chang JS, Herrmann C, Xia A (eds) Bioreactors for microbial biomass and energy conversion. Springer, Singapore, pp 117–162
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597
Neilson AH, Lewin RA (1974) The uptake and utilization of organic carbon by algae: an essay in comparative biochemistry. Phycologia 13:227–264
O’Grady J, Morgan JA (2011) Heterotrophic growth and lipid production of Chlorella protothecoides on glycerol. Bioprocess Biosyst Eng 34:121–125
Pagnanelli F, Altimari P, Trabucco F, Toro L (2014) Mixotrophic growth of Chlorella vulgaris and Nannochloropsis oculata: interaction between glucose and nitrate. J Chem Technol Biotechnol 89:652–661
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Parker BC, Bold HC, Deason TR (1961) Facultative heterotrophy in some Chlorococcacean algae. Science 133:761–763
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Piorreck M, Baasch KH, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater green and blue-green algae under different nitrogen regimes. Phytochemistry 23:207–216
Přibyl P, Cepák V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella, and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Procházková G, Brányiková I, Zachleder V, Brányik T (2014) Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J Appl Phycol 26:1359–1377
Reitan KI, Rainuzzo JR, Olsen Y (1994) Effect of nutrient limitation on fatty acid and lipid content of marine microalgae. J Phycol 30:972–979
Rhee GY (1978) Effects of N:P atomic ratios and nitrate limitation on algal growth, cell composition, and nitrate uptake. Limnol Oceanogr 23:10–25
Richardson B, Orcutt DM, Schwertner HA, Martinez CL, Wickline HE (1969) Effects of nitrogen limitation on the growth and composition of unicellular algae in continuous culture. Appl Microbiol 18:245–250
Richardson JW, Johnson MD, Outlaw JL (2012) Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res 1:93–100
Roach BMB (1926) On the relation of certain soil algae to some soluble carbon compounds. Ann Bot 40:149–201
Roach BMB (1927) On the carbon nutrition of some algae isolated from soil. Ann Bot 41:509–517
Rodhe W (1953) Can plankton production continue during winter darkness in subarctic lakes? Verh Intern Ver Limnol 12:117–122
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Ruangsomboon S, Ganmanee M, Choochote S (2013) Effects of different nitrogen, phosphorus, and iron concentrations and salinity on lipid production in newly isolated strain of the tropical green microalga, Scenedesmus dimorphus KMITL. J Appl Phycol 25:867–874
Schneegurt MA, Sherman DM, Sherman LA (1997) Growth, physiology, and ultrastructure of a diazotrophic cyanobacterium, Cyanothece sp. strain ATCC 51142, in mixotrophic and chemoheterotrophic cultures. J Phycol 33:632–642
Scott TA Jr, Melvin EH (1953) Determination of dextran with anthrone. Anal Chem 25:1656–1661
Seifter S, Dayton S, Novic B, Muntwyler E (1950) The estimation of glycogen with the anthrone reagent. Arch Biochem 25:191–200
Seilheimer JA, Jackson DF (1963) A comparison of algal growth in cultures exposed and unexposed to solar radiation. Trans Am Microsc Soc 82:78–83
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: interspecific differences and effects of nitrate, silicate and light-dark cycles. J Phycol 17:374–384
Shrestha N, Schneegurt MA (2016) Phosphorus limitation enhances biodiesel production in dark-grown algae fed grain or lignocellulosic hydrolysates. Abstracts and Program, 116th Annual Meeting of the American Society for Microbiology, Boston, June 2016
Singh P, Kumari S, Guldhe A, Misra R, Rawat I, Bux F (2016) Trends and novel strategies for enhancing lipid accumulation and quality in microalgae. Renew Sust Energ Rev 55:1–16
Skinner CE, Gardner CG (1930) The utilization of nitrogenous organic compounds and sodium salts of organic acids by certain soil algae in darkness and in the light. J Bacteriol 19:161–179
Smith VH, Sturm BSM, deNoyelles FJ, Billings SA (2010) The ecology of algal biodiesel production. Trends Ecol Evol 25:301–309
Sturm BSM, Peltier E, Smith V, deNoyelles F (2011) Controls of microalgal biomass and lipid production in municipal wastewater-fed bioreactors. Env Progr Sustain Energ 31:10–16
Su G, Jiao K, Li Z, Guo X, Chang J, Ndikubwimana T, Sun Y, Zeng X, Lu Y, Lin L (2016) Phosphate limitation promotes unsaturated fatty acids and arachidonic acid biosynthesis by microalgae Porphyridium purpureum. Bioprocess Biosyst Eng 39:1129–1136
Verma NM, Mehrotra S, Shukla A, Mishra BN (2010) Prospective of biodiesel production utilizing microalgae as the cell factories: a comprehensive discussion. Afr J Biotechnol 9:1402–1411
Wagley PK, Schneegurt MA (2012a) Microbial community analysis of open ponds for algal biodiesel production. In: Proceedings: 8th annual symposium: graduate research and scholarly projects. Wichita, KS: Wichita State University, pp 145–146
Wagley PK, Schneegurt MA (2012b) Molecular analysis of microbial community structure in open ponds for algal biodiesel production. Trans KS Acad Sci 115:78
Wan M, Liu P, Xia J, Rosenberg JN, Oyler GA, Betenbaugh MJ, Nie Z, Qiu G (2011) The effect of mixotrophy on microalgal growth, lipid content, and expression levels of three pathway genes in Chlorella sorokiniana. Appl Microbiol Biotechnol 91:835–844
Wu QY, Yin S, Sheng GY, Fu JM (1994) New discoveries in study on hydrocarbons from thermal degradation of heterotrophically yellowing algae. Sci China Ser B 37:326–335
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Xu L, Weathers PJ, Xiong XR, Liu CZ (2009) Microalgal bioreactors: challenges and opportunities. Eng Life Sci 3:178–189
Acknowledgments
The authors appreciate the technical support of James Crisler, Timothy Eberl, Casper Fredsgaard, Devon Miller, and Robert Tolley. We thank Belinda Sturm (University of Kansas) for performing lipid analyses. Biomass hydrolysates were kindly provided by Donghai Wang and Ke Zhang (Kansas State University) and Accelerase enzyme was a gift from Stephen Crawford, Deborah Dodge, and Chris Nguyen (DuPont). Special thanks to Gregory Houseman and Leland Russell for collecting Big Bluestem biomass. Preliminary accounts of this work have been presented previously and abstracted (Dandinpet and Schneegurt 2013a, b, 2014; Shrestha and Schneegurt 2016). This is publication no. 33 in the series of reports from the Wichita State University Biological Field Station.
Funding
This work was supported by an award from Kansas National Science Foundation (NSF) Established Program to Stimulate Competitive Research (EPSCoR) (EPS-0903806). Additional student support was from Kansas Institutional Development Award (IDeA) Networks of Biomedical Research Excellence (KINBRE) of the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) (P20 GM103418).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shrestha, N., Dandinpet, K.K. & Schneegurt, M.A. Effects of nitrogen and phosphorus limitation on lipid accumulation by Chlorella kessleri str. UTEX 263 grown in darkness. J Appl Phycol 32, 2795–2805 (2020). https://doi.org/10.1007/s10811-020-02144-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02144-x