Abstract
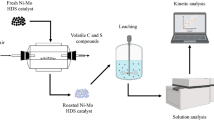
In this present paper, chelating extraction of metals from spent hydrodesulphurization catalyst was carried out using ethylene diamine tetraacetic acid as complexing agent. Mo, Ni and Co metals were precipitated in ammonium molybdate, nickel dimethylglyoxime and cobalt hydroxide forms at pH:2, pH:6 and pH:10, respectively. The highest metal extraction yields (90.22% Mo, 96.71% Co, 95.31% Ni and 19.98% Al) were achieved under optimum process conditions. The activation energy values (Ea) of Co, Mo and Ni were calculated as 14.36 kJ/mol, 16.85 kJ/mol and 15.93 kJ/mol, respectively. It was determined that leaching kinetics fitted to the pseudo-first homogenous model and the chelating process was controlled by diffusion mechanism. In the light of the kinetic data, the kinetic equation including the process parameters was obtained as follows: \(\ln \left( {1 - x} \right) = 1.217 \times 10^{ - 4} [(C_{A} )^{1.068} \left( D \right)^{ - 0.929} (K/S)^{ - 0.850} (R)^{0.185} \exp ( - 6462.6/T)] t\). The results provided a new approach both for reducing the solid waste load of the petrochemical industry and for efficient recovery of metals from the spent hydrodesulphurization catalyst using EDTA.











Similar content being viewed by others
References
A. Akcil, F. Vegliò, F. Ferella, M. D. Okudan, and A. Tuncuk, Waste Manag. 45, 420 (2015).
M. Marafi and A. Stanislaus, Resour. Conserv. Recycl. 52, 859 (2008).
M. Marafi and A. Stanislaus, Resour. Conserv. Recycl. 53, 1 (2008).
P. Dufresne, Appl. Catal. A Gen. 322, 67 (2007).
C. Liu, Y. Yu, and H. Zhao, Fuel Process. Technol. 86, 449 (2005).
L. E. Macaskie, I. P. Mikheenko, P. Yong, K. Deplanche, A. J. Murray, M. Paterson-Beedle, V. S. Coker, C. I. Pearce, R. Cutting, and R. A. D. Pattrick, Hydrometallurgy 104, 483 (2010).
A. Marafi, S. Fukase, M. Al-Marri, and A. Stanislaus, Energy & Fuels 17, 661 (2003).
H. Srichandan, S. Singh, K. Blight, A. Pathak, D. J. Kim, S. Lee, and S. W. Lee, Int. J. Miner. Process. 134, 66 (2015).
M. Motaghed, S. M. Mousavi, S. O. Rastegar, and S. A. Shojaosadati, Bioresour. Technol. 171, 401 (2014).
J. Ramos-Cano, G. González-Zamarripa, F. R. Carrillo-Pedroza, M. de Jesús Soria-Aguilar, A. Hurtado-Macías, and A. Cano-Vielma, Int. J. Miner. Process. 148, 41 (2016).
S. Huang, J. Liu, C. Zhang, B. Hu, X. Wang, M. Wang, and X. Wang, JOM 71, 4681 (2019).
N. Nagar, H. Garg, and C. S. Gahan, Biocatal. Agric. Biotechnol. 20, 101252 (2019).
S. Vyas and Y.-P. Ting, Chemosphere 160, 7 (2016).
B. B. Kar, P. Datta, and V. N. Misra, Hydrometallurgy 72, 87 (2004).
B. B. Kar, B. V. R. Murthy, and V. N. Misra, Int. J. Miner. Process. 76, 143 (2005).
M. H. Shariat, N. Setoodeh, and R. A. Dehghan, Miner. Eng. 14, 815 (2001).
D. D. Sun, J. H. Tay, H. K. Cheong, D. L. K. Leung, and G. Qian, J. Hazard. Mater. 87, 213 (2001).
D. Mishra, D.-J. Kim, J.-G. Ahn, and Y.-H. Rhee, Met. Mater. Int. 11, 249 (2005).
G. Chauhan, K. K. Pant, and K. D. P. Nigam, Ind. Eng. Chem. Res. 52, 16724 (2013).
D. Mishra, G. R. Chaudhury, D. J. Kim, and J. G. Ahn, Hydrometallurgy 101, 35 (2010).
W. Mulak, A. Szymczycha, A. Lesniewicz, and W. Zyrnicki, Physicochem. Probl. Miner. Process. 40, 69 (2006).
K. Mazurek, K. Białowicz, and M. Trypuć, Hydrometallurgy 103, 19 (2010).
F. Beolchini, V. Fonti, F. Ferella, and F. Vegliò, J. Hazard. Mater. 178, 529 (2010).
Y.-C. Lai, W.-J. Lee, K.-L. Huang, and C.-M. Wu, J. Hazard. Mater. 154, 588 (2008).
I. M. Valverde Jr, J. F. Paulino, and J. C. Afonso, J. Hazard. Mater. 160, 310 (2008).
K. H. Park, D. Mohapatra, and B. R. Reddy, J. Hazard. Mater. 138, 311 (2006).
D. Santhiya and Y.-P. Ting, J. Biotechnol. 121, 62 (2006).
K. H. Park, B. R. Reddy, D. Mohapatra, and C.-W. Nam, Int. J. Miner. Process. 80, 261 (2006).
D. Santhiya and Y.-P. Ting, J. Biotechnol. 116, 171 (2005).
S. P. Barik, K.-H. Park, P. K. Parhi, and J. T. Park, Hydrometallurgy 111, 46 (2012).
H. Arslanoğlu and A. Yaraş, Pet. Sci. Technol. 1 (2019).
H.-I. Kim, K.-H. Park, and D. Mishra, Hydrometallurgy 98, 192 (2009).
A. J. Chaudhary, J. D. Donaldson, S. C. Boddington, and S. M. Grimes, Hydrometallurgy 34, 137 (1993).
A. Szymczycha-Madeja, J. Hazard. Mater. 186, 2157 (2011).
T. E. Norgate, S. Jahanshahi, and W. J. Rankin, J. Clean. Prod. 15, 838 (2007).
G. Chauhan, K. K. Pant, and K. D. P. Nigam, Environ. Sci. Process. Impacts 17, 12 (2015).
J. Yang, Q. Zeng, L. Peng, M. Lei, H. Song, B. Tie, and J. Gu, J. Environ. Sci. 25, 413 (2013).
S. Zhang, Z. Yang, B. Wu, Y. Wang, R. Wu, and Y. Liao, CLEAN–Soil, Air, Water 42, 641 (2014).
A. C. Garrabrants and D. S. Kosson, Waste Manag. 20, 155 (2000).
D. C. W. Tsang, W. Zhang, and I. M. C. Lo, Chemosphere 68, 234 (2007).
W. Zhang, D. C. W. Tsang, and I. M. C. Lo, Chemosphere 66, 2025 (2007).
W. Xia, H. Gao, X. Wang, C. Zhou, Y. Liu, T. Fan, and X. Wang, J. Hazard. Mater. 164, 936 (2009).
W. Zhang, L. Tong, Y. Yuan, Z. Liu, H. Huang, F. Tan, and R. Qiu, J. Hazard. Mater. 178, 578 (2010).
R. S. Tejowulan and W. H. Hendershot, Environ. Pollut. 103, 135 (1998).
C. E. Martınez and H. L. Motto, Environ. Pollut. 107, 153 (2000).
S. Goel, K. K. Pant, and K. D. P. Nigam, J. Hazard. Mater. 171, 253 (2009).
K. R. Vuyyuru, K. K. Pant, V. V Krishnan, and K. D. P. Nigam, Ind. Eng. Chem. Res. 49, 2014 (2010).
O. Alpaslan, A. Yaras, H. Arslanoglu, Bartın Univ. J. Eng. Technol. Sci. 7, 18 (2019).
B. Nowack, Environ. Sci. Technol. 36, 4009 (2002).
T. C. M. Yip, D. C. W. Tsang, and I. M. C. Lo, Chemosphere 81, 415 (2010).
I. M. C. Lo, D. C. W. Tsang, T. C. M. Yip, F. Wang, and W. Zhang, Chemosphere 83, 7 (2011).
W. Zhang and D. C. W. Tsang, Chemosphere 91, 1281 (2013).
G. Chauhan, K. K. Pant, and K. D. P. Nigam, Ind. Eng. Chem. Res. 51, 10354 (2012).
J. Singh and B.-K. Lee, Process Saf. Environ. Prot. 99, 69 (2016).
S. Rao, T. Yang, D. Zhang, W. Liu, L. Chen, Z. Hao, Q. Xiao, and J. Wen, Hydrometallurgy 158, 101 (2015).
C. Kim, Y. Lee, and S. K. Ong, Chemosphere 51, 845 (2003).
A. Verma and S. Hait, Process Saf. Environ. Prot. 121, 1 (2019).
P. Jadhao, G. Chauhan, K. K. Pant, and K. D. P. Nigam, Waste Manag. 57, 102 (2016).
Z. Wang, S. Guo, and C. Ye, Procedia Environ. Sci. 31, 917 (2016).
M. Authier-Martin, G. Forte, S. Ostap, and J. See, JOM 53, 36 (2001).
M. Goto, B. C. Roy, and T. Hirose, J. Supercrit. Fluids 9, 128 (1996).
Kn. C. Liddell, Hydrometallurgy 79, 62 (2005).
C. Y. Wen, Ind. Eng. Chem. 60, 34 (1968).
O. Levenspiel, Inc., New York (1972).
A. Ekmekyapar, E. Aktaş, A. Künkül, and N. Demirkiran, Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 43, 764 (2012).
Acknowledgements
This paper was produced from Orhon ALPASLAN’s master thesis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alpaslan, O., Yaras, A. & Arslanoğlu, H. A Kinetic Model for Chelating Extraction of Metals from Spent Hydrodesulphurization Catalyst by Complexing Agent. Trans Indian Inst Met 73, 1925–1937 (2020). https://doi.org/10.1007/s12666-020-02007-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12666-020-02007-6