Abstract
FGF23 is a hormone produced by osteocytes in response to an elevation in the concentration of extracellular phosphate. Excess production of FGF23 by bone cells, or rarely by tumors, is the hormonal basis for several musculoskeletal syndromes characterized by hypophosphatemia due to renal phosphate wasting. FGF23-dependent chronic hypophosphatemia causes rickets and osteomalacia, as well as other skeletal complications. Genetic disorders of FGF23-mediated hypophosphatemia include X-linked hypophosphatemia (XLH), autosomal dominant hypophosphatemic rickets (ADHR), autosomal recessive hypophosphatemic rickets (ARHR), fibrous dysplasia of bone, McCune-Albright syndrome, and epidermal nevus syndrome (ENS), also known as cutaneous skeletal hypophosphatemia syndrome (CSHS). The principle acquired form of FGF23-mediated hypophosphatemia is tumor-induced osteomalacia (TIO). This review summarizes current knowledge about the pathophysiology and clinical presentation of the most common FGF23-mediated conditions, with a focus on new treatment modalities. For many decades, calcitriol and phosphate supplements were the mainstay of therapy. Recently, burosumab, a monoclonal blocking antibody to FGF23, has been approved for treatment of XLH in children and adults, and an active comparator trial in children has shown good efficacy and safety for this drug. The remainder of FGF23-mediated hypophosphatemic disorders continue to be treated with phosphate and calcitriol, although ongoing trials with burosumab for treatment of tumor-induced osteomalacia show early promise. Burosumab may be an effective treatment for the remainder of FGF23-mediated disorders, but clinical trials to support that possibility are at present not available.


Adapted from Imel, E.A. et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open label, phase 3 trial. Lancet (London, England) 393: 2416–2427 (2019)

Adapted from Insogna, K.L. et al. A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial Evaluating the Efficacy of Burosumab, an Anti-FGF23 Antibody, in Adults With X-Linked Hypophosphatemia: Week 24 Primary Analysis. Journal of Bone and Mineral Research 33:1383–1393 (2018)

Adapted from Portale, A.A. et al. Continued Beneficial Effects of Burosumab in Adults with X-Linked Hypophosphatemia: Results from a 24-Week Treatment Continuation Period After a 24-Week Double-Blind Placebo-Controlled Period. Calcified Tissue International 105: 271–284 (2019)

Adapted from Insogna, K.L., et al., Burosumab Improved Histomorphometric Measures of Osteomalacia in Adults with X-Linked Hypophosphatemia: A Phase 3, Single-Arm, International Trial. J Bone Miner Res, 2019. 34(12): p. 2183–2191

Adapted from Wolf, M. & White, K.E. Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Current Opinion in Nephrology and Hypertension 23: 411–419 (2014)

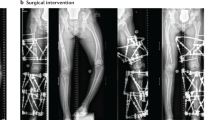
Adapted from Florenzano, P., R.I. Gafni, and M.T. Collins, Tumor-induced osteomalacia. Bone Reports, 2017. 7: p. 90–97

Adapted from El-Maouche, D., et al., (68)Ga-DOTATATE for Tumor Localization in Tumor-Induced Osteomalacia. The Journal of Clinical Endocrinology and Metabolism, 2016. 101(10): p. 3575–3581
Similar content being viewed by others
References
Chong WH et al (2011) Tumor-induced osteomalacia. Endocr Relat Cancer 18(3):R53–R77
Minisola S et al (2017) Tumour-induced osteomalacia. Nature reviews Disease primers 3:17044–17044
Glorieux FH et al (1980) Bone response to phosphate salts, ergocalciferol, and calcitriol in hypophosphatemic vitamin D-resistant rickets. N Engl J Med 303(18):1023–1031
Carpenter TO et al (2018) Burosumab Therapy in Children with X-Linked Hypophosphatemia. N Engl J Med 378(21):1987–1998
Imel EA et al (2019) Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet 393(10189):2416–2427
Imel EA et al (2015) Prolonged Correction of Serum Phosphorus in Adults With X-Linked Hypophosphatemia Using Monthly Doses of KRN23. The Journal of clinical endocrinology and metabolism 100(7):2565–2573
Insogna KL et al (2018) A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial Evaluating the Efficacy of Burosumab, an Anti-FGF23 Antibody, in Adults With X-Linked Hypophosphatemia: Week 24 Primary Analysis. J Bone Miner Res 33(8):1383–1393
Insogna KL et al (2019) Burosumab Improved Histomorphometric Measures of Osteomalacia in Adults with X-Linked Hypophosphatemia: A Phase 3, Single-Arm. International Trial J Bone Miner Res 34(12):2183–2191
Portale AA et al (2019) Continued Beneficial Effects of Burosumab in Adults with X-Linked Hypophosphatemia: Results from a 24-Week Treatment Continuation Period After a 24-Week Double-Blind Placebo-Controlled Period. Calcif Tissue Int 105(3):271–284
Ruppe MD et al (2016) Effect of four monthly doses of a human monoclonal anti-FGF23 antibody (KRN23) on quality of life in X-linked hypophosphatemia. Bone reports 5:158–162
Jan de Beur, S., et al., Burosumab improves the biochemical, skeletal, and clinical symptoms of tumor-induced osteomalacia (TIO) syndrome, in World Congress on Osteoporosis, Osteoarthritis and Musculoskeletal Diseases. 2019: Paris, France.
Bhattacharyya N et al (2012) Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab 23(12):610–618
Tagliabracci VS et al (2014) Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci U S A 111(15):5520–5525
Erben RG (2016) Update on FGF23 and Klotho signaling. Mol Cell Endocrinol 432:56–65
Andrukhova O et al (2012) FGF23 acts directly on renal proximal tubules to induce phosphaturia through activation of the ERK1/2-SGK1 signaling pathway. Bone 51(3):621–628
Shimada T et al (2004) Targeted ablation of Fgf23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D metabolism. J Clin Invest 113(4):561–568
Albright F, Butler AM, Bloomberg E (1937) RICKETS RESISTANT TO VITAMIN D THERAPY. Am J Dis Child 54(3):529–547
Eicher EM et al (1976) Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci U S A 73(12):4667–4671
Morgan JM et al (1974) Renal transplantation in hypophosphatemia with vitamin D-resistant rickets. Arch Intern Med 134(3):549–552
Meyer RA Jr, Meyer MH, Gray RW (1989) Parabiosis suggests a humoral factor is involved in X-linked hypophosphatemia in mice. J Bone Miner Res 4(4):493–500
A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets (1995) The HYP Consortium. Nat Genet 11(2):130–136
White KE, Evans WE (2000) Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26(3):345–348
Shimada T et al (2001) Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci U S A 98(11):6500–6505
Jonsson KB et al (2003) Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348(17):1656–1663
Yamazaki Y et al (2002) Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab 87(11):4957–4960
Addison WN et al (2010) Phosphorylation-dependent inhibition of mineralization by osteopontin ASARM peptides is regulated by PHEX cleavage. J Bone Miner Res 25(4):695–705
Barros NM et al (2013) Proteolytic processing of osteopontin by PHEX and accumulation of osteopontin fragments in Hyp mouse bone, the murine model of X-linked hypophosphatemia. J Bone Miner Res 28(3):688–699
David V et al (2011) ASARM peptides: PHEX-dependent and -independent regulation of serum phosphate. Am J Physiol Renal Physiol 300(3):F783–F791
Beck-Nielsen SS et al (2009) Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol 160(3):491–497
Rafaelsen S et al (2016) Hereditary hypophosphatemia in Norway: a retrospective population-based study of genotypes, phenotypes, and treatment complications. Eur J Endocrinol 174(2):125–136
Ruppe, M.D., X-Linked Hypophosphatemia, in GeneReviews (Internet), M.P. Adam, et al., Editors. 1993, University of Washington, Seattle; 1993–2019.: Seattle (WA).
Rothenbuhler A et al (2019) High Incidence of Cranial Synostosis and Chiari I Malformation in Children With X-Linked Hypophosphatemic Rickets (XLHR). J Bone Miner Res 34(3):490–496
Turan S et al (2010) Identification of a novel dentin matrix protein-1 (DMP-1) mutation and dental anomalies in a kindred with autosomal recessive hypophosphatemia. Bone 46(2):402–409
Pesta DH et al (2016) Hypophosphatemia promotes lower rates of muscle ATP synthesis. Faseb j 30(10):3378–3387
Connor J et al (2015) Conventional Therapy in Adults With X-Linked Hypophosphatemia: Effects on Enthesopathy and Dental Disease. J Clin Endocrinol Metab 100(10):3625–3632
Liang G et al (2009) Survey of the enthesopathy of X-linked hypophosphatemia and its characterization in Hyp mice. Calcif Tissue Int 85(3):235–246
Hirao Y et al (2016) Extensive ossification of the paraspinal ligaments in a patient with vitamin D-resistant rickets: Case report with literature review. Int J Surg Case Rep 27:125–128
Shiba M et al (2015) Cervical ossification of posterior longitudinal ligament in x-linked hypophosphatemic rickets revealing homogeneously increased vertebral bone density. Asian Spine J 9(1):106–109
Chesher D et al (2018) Outcome of adult patients with X-linked hypophosphatemia caused by PHEX gene mutations. J Inherit Metab Dis 41(5):865–876
Fishman G et al (2004) Hearing impairment in familial X-linked hypophosphatemic rickets. Eur J Pediatr 163(10):622–623
Carpenter TO et al (2011) A clinician's guide to X-linked hypophosphatemia. J Bone Miner Res 26(7):1381–1388
Carpenter TO et al (1994) Nocturnal hyperparathyroidism: a frequent feature of X-linked hypophosphatemia. J Clin Endocrinol Metab 78(6):1378–1383
Baroncelli GI et al (2001) Effect of growth hormone treatment on final height, phosphate metabolism, and bone mineral density in children with X-linked hypophosphatemic rickets. J Pediatr 138(2):236–243
Haffner D et al (2004) Effects of growth hormone treatment on body proportions and final height among small children with X-linked hypophosphatemic rickets. Pediatrics 113(6):e593–e596
Zivicnjak M et al (2011) Three-year growth hormone treatment in short children with X-linked hypophosphatemic rickets: effects on linear growth and body disproportion. J Clin Endocrinol Metab 96(12):E2097–E2105
Alon U, Chan JC (1985) Effects of hydrochlorothiazide and amiloride in renal hypophosphatemic rickets. Pediatrics 75(4):754–763
Seikaly MG, Baum M (2001) Thiazide diuretics arrest the progression of nephrocalcinosis in children with X-linked hypophosphatemia. Pediatrics 108(1):E6
Carpenter TO et al (1996) 24,25 Dihydroxyvitamin D supplementation corrects hyperparathyroidism and improves skeletal abnormalities in X-linked hypophosphatemic rickets–a clinical research center study. J Clin Endocrinol Metab 81(6):2381–2388
Sullivan W et al (1992) A prospective trial of phosphate and 1,25-dihydroxyvitamin D3 therapy in symptomatic adults with X-linked hypophosphatemic rickets. J Clin Endocrinol Metab 75(3):879–885
Carpenter TO et al (2014) Effect of paricalcitol on circulating parathyroid hormone in X-linked hypophosphatemia: a randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 99(9):3103–3111
Imel EA et al (2015) Prolonged Correction of Serum Phosphorus in Adults With X-Linked Hypophosphatemia Using Monthly Doses of KRN23. J Clin Endocrinol Metab 100(7):2565–2573
Ruppe MD et al (2016) Effect of four monthly doses of a human monoclonal anti-FGF23 antibody (KRN23) on quality of life in X-linked hypophosphatemia. Bone Rep 5:158–162
Benet-Pages A et al (2004) FGF23 is processed by proprotein convertases but not by PHEX. Bone 35(2):455–462
Econs MJ, McEnery PT (1997) Autosomal dominant hypophosphatemic rickets/osteomalacia: clinical characterization of a novel renal phosphate-wasting disorder. J Clin Endocrinol Metab 82(2):674–681
Wolf M, White KE (2014) Coupling fibroblast growth factor 23 production and cleavage: iron deficiency, rickets, and kidney disease. Curr Opin Nephrol Hypertens 23(4):411–419
Imel, E.A., et al., Oral Iron Replacement Normalizes Fibroblast Growth Factor 23 in Iron-Deficient Patients With Autosomal Dominant Hypophosphatemic Rickets. J Bone Miner Res, 2019.
Steichen-Gersdorf E et al (2015) Early onset hearing loss in autosomal recessive hypophosphatemic rickets caused by loss of function mutation in ENPP1. J Pediatr Endocrinol Metab 28(7–8):967–970
Feng JQ et al (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38(11):1310–1315
Ichikawa S et al (2017) A Mutation in the Dmp1 Gene Alters Phosphate Responsiveness in Mice. Endocrinology 158(3):470–476
Folpe AL et al (2004) Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol 28(1):1–30
Carpenter TO et al (2005) Fibroblast growth factor 7: an inhibitor of phosphate transport derived from oncogenic osteomalacia-causing tumors. J Clin Endocrinol Metab 90(2):1012–1020
De Beur SM et al (2002) Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res 17(6):1102–1110
Florenzano P, Gafni RI, Collins MT (2017) Tumor-induced osteomalacia Bone reports 7:90–97
Nakahama H et al (1995) Prostate cancer-induced oncogenic hypophosphatemic osteomalacia. Urol Int 55(1):38–40
Narvaez J et al (2005) Acquired hypophosphatemic osteomalacia associated with multiple myeloma. Joint Bone Spine 72(5):424–426
Sanders, L., The young woman was a healthy and avid runner. Now she could barely walk. Why?, in The New York Times. 2018: New York, NY. p. 20.
Jan De Beur S et al (2019) OR13–1 Burosumab Improves the Biochemical, Skeletal, and Clinical Symptoms of Tumor-Induced Osteomalacia Syndrome. Journal of the Endocrine Society 2019:2019. https://doi.org/10.1210/js.2019-OR13-1
Lee JC et al (2015) Identification of a novel FN1-FGFR1 genetic fusion as a frequent event in phosphaturic mesenchymal tumour. J Pathol 235(4):539–545
El-Maouche D et al (2016) (68)Ga-DOTATATE for Tumor Localization in Tumor-Induced Osteomalacia. The Journal of clinical endocrinology and metabolism 101(10):3575–3581
Jan de Beur SM et al (2002) Localisation of mesenchymal tumours by somatostatin receptor imaging. Lancet 359(9308):761–763
Hesse E et al (2007) Oncogenic osteomalacia: exact tumor localization by co-registration of positron emission and computed tomography. J Bone Miner Res 22(1):158–162
Hesse E, Rosenthal H, Bastian L (2007) Radiofrequency ablation of a tumor causing oncogenic osteomalacia. N Engl J Med 357(4):422–424
Wild D et al (2005) 68Ga-DOTANOC: a first compound for PET imaging with high affinity for somatostatin receptor subtypes 2 and 5. Eur J Nucl Med Mol Imaging 32(6):724
Zhang J et al (2015) 68Ga DOTATATE PET/CT is an Accurate Imaging Modality in the Detection of Culprit Tumors Causing Osteomalacia. Clin Nucl Med 40(8):642–646
Bhavani N et al (2016) Utility of Gallium-68 DOTANOC PET/CT in the localization of Tumour-induced osteomalacia. Clin Endocrinol 84(1):134–140
Rayamajhi SJ et al (2019) Tumor-induced osteomalacia - Current imaging modalities and a systematic approach for tumor localization. Clin Imaging 56:114–123
Maybody M et al (2016) Ga-68 DOTATOC PET/CT-Guided Biopsy and Cryoablation with Autoradiography of Biopsy Specimen for Treatment of Tumor-Induced Osteomalacia. Cardiovasc Intervent Radiol 39(9):1352–1357
Basu S, Fargose P (2016) 177Lu-DOTATATE PRRT in Recurrent Skull-Base Phosphaturic Mesenchymal Tumor Causing Osteomalacia: A Potential Application of PRRT Beyond Neuroendocrine Tumors. Journal of nuclear medicine technology 44(4):248–250
Chong WH et al (2011) The importance of whole body imaging in tumor-induced osteomalacia. The Journal of clinical endocrinology and metabolism 96(12):3599–3600
Collins, M.T., et al., Striking Response of Tumor-Induced Osteomalacia to the FGFR Inhibitor NVP-BGJ398. J Bone Miner Res, 2015. 28(Supplement 1): p. Available at https://www.asbmr.org/education/AbstractDetail?aid=c5464be6-d873-49f3-bb71-719e2198867e. Accessed January 8, 2020.
Baia LC et al (2015) Phosphate and FGF-23 homeostasis after kidney transplantation. Nat Rev Nephrol 11(11):656–666
Okada M et al (1982) 2 cases of nonspecific multiple ulcers of the small intestine associated with osteomalacia caused by long-term intravenous administration of saccharated ferric oxide. Nihon Naika Gakkai Zasshi 71(11):1566–1572
Okada M et al (1983) Hypophosphatemia induced by intravenous administration of Saccharated iron oxide. Klin Wochenschr 61(2):99–102
Sato K et al (1997) Saccharated ferric oxide (SFO)-induced osteomalacia: in vitro inhibition by SFO of bone formation and 1,25-dihydroxy-vitamin D production in renal tubules. Bone 21(1):57–64
Sato K, Shiraki M (1998) Saccharated ferric oxide-induced osteomalacia in Japan: iron-induced osteopathy due to nephropathy. Endocr J 45(4):431–439
Schouten BJ et al (2009) Iron polymaltose-induced FGF23 elevation complicated by hypophosphataemic osteomalacia. Ann Clin Biochem 46(Pt 2):167–169
Yamamoto S et al (2012) Fibroblast growth factor 23-related osteomalacia caused by the prolonged administration of saccharated ferric oxide. Intern Med 51(17):2375–2378
Imamura K (1984) Effects of intravenous administration of iron preparations on the metabolism of phosphorus Comparative study on 3 iron preparations. Fukuoka Igaku Zasshi 75(6):316–326
Schaefer B et al (2016) Choice of High-Dose Intravenous Iron Preparation Determines Hypophosphatemia Risk. PLoS ONE 11(12):e0167146
Felsenfeld AJ et al (1986) Hypophosphatemia in long-term renal transplant recipients: effects on bone histology and 1,25-dihydroxycholecalciferol. Miner Electrolyte Metab 12(5–6):333–341
Levi M (2001) Post-transplant hypophosphatemia. Kidney Int 59(6):2377–2387
Moorhead JF et al (1974) Hypophosphataemic osteomalacia after cadaveric renal transplantation. Lancet 1(7860):694–697
Rosenbaum RW et al (1981) Decreased phosphate reabsorption after renal transplantation: Evidence for a mechanism independent of calcium and parathyroid hormone. Kidney Int 19(4):568–578
Evenepoel P et al (2007) Tertiary 'hyperphosphatoninism' accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant 7(5):1193–1200
Cianciolo G, Cozzolino M (2016) FGF23 in kidney transplant: the strange case of Doctor Jekyll and Mister Hyde. Clin Kidney J 9(5):665–668
Alshayeb HM, Josephson MA, Sprague SM (2013) CKD-mineral and bone disorder management in kidney transplant recipients. Am J Kidney Dis 61(2):310–325
Cianciolo G et al (2016) Vitamin D in Kidney Transplant Recipients: Mechanisms and Therapy. Am J Nephrol 43(6):397–407
Nafidi O et al (2009) Mechanisms of renal phosphate loss in liver resection-associated hypophosphatemia. Ann Surg 249(5):824–827
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Diana Athonvarangkul declares that she has no conflict of interest. Karl L. Insogna has received grants from and been a consultant for Ultragenyx Pharmaceutical.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Athonvarangkul, D., Insogna, K.L. New Therapies for Hypophosphatemia-Related to FGF23 Excess. Calcif Tissue Int 108, 143–157 (2021). https://doi.org/10.1007/s00223-020-00705-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00705-3