Abstract
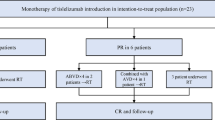
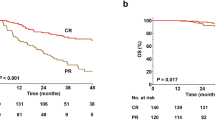
Classical Hodgkin lymphoma (cHL) is considered a curable disease; however, in approximately one-third of the responding patients, the disease relapses following completion of therapy. One of the drugs that have been approved for the treatment of relapsed/refractory cHL is nivolumab, an immune check point inhibitor that shows its effects by blocking the programmed death 1 (PD-1) receptor. In this study, we present a retrospective “real-life” analysis of the usage of nivolumab in patients with relapsed/refractory cHL that have joined the named patient program (NPP) for nivolumab, reflecting 4 years of experience in the treatment of relapsed/refractory cHL. We present a retrospective analysis of 87 patients (median age, 30) that participated in the NPP in 24 different centers, who had relapsed/refractory cHL and were consequently treated with nivolumab. The median follow-up was 29 months, and the median number of previous treatments was 5 (2–11). In this study, the best overall response rate was 70% (CR, 36%; PR, 34%). Twenty-eight of the responding patients underwent subsequent stem cell transplantation (SCT). Among 15 patients receiving allogeneic stem cell transplantation, 9 patients underwent transplantation with objective response, of which 8 of them are currently alive with ongoing response. At the time of analysis, 23 patients remained on nivolumab treatment and the rest discontinued therapy. The main reason for discontinuing nivolumab was disease progression (n = 23). The safety profile was acceptable, with only nine patients requiring cessation of nivolumab due to serious adverse events. The 24-month progression-free and overall survival rates were 58.5% (95% CI, 0.47–0.68) and 78.7% (95% CI, 0.68–0.86), respectively. Eighteen patients died during the follow-up and only one of these was regarded to be treatment-related. With its efficacy and its safety profile, PD-1 blockers became an important treatment option in the heavily pretreated cHL patients.



Similar content being viewed by others
References
Canellos GP, Anderson JR, Propert KJ, Nissen N, Cooper MR, Henderson ES, Green MR, Gottlieb A, Peterson BA (1992) Chemotherapy of advanced Hodgkin’s disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med 327(21):1478–1484. https://doi.org/10.1056/NEJM199211193272102
Viviani S, Zinzani PL, Rambaldi A, Brusamolino E, Levis A, Bonfante V, Vitolo U, Pulsoni A, Liberati AM, Specchia G, Valagussa P, Rossi A, Zaja F, Pogliani EM, Pregno P, Gotti M, Gallamini A, Rota Scalabrini D, Bonadonna G, Gianni AM, Michelangelo F, Gruppo Italiano di Terapie Innovative nei L, Intergruppo Italiano L (2011) ABVD versus BEACOPP for Hodgkin’s lymphoma when high-dose salvage is planned. N Engl J Med 365(3):203–212. https://doi.org/10.1056/NEJMoa1100340
Borchmann P, Haverkamp H, Lohri A, Mey U, Kreissl S, Greil R, Markova J, Feuring-Buske M, Meissner J, Duhrsen U, Ostermann H, Keller U, Maschmeyer G, Kuhnert G, Dietlein M, Kobe C, Eich H, Baues C, Stein H, Fuchs M, Diehl V, Engert A (2017) Progression-free survival of early interim PET-positive patients with advanced stage Hodgkin’s lymphoma treated with BEACOPPescalated alone or in combination with rituximab (HD18): an open-label, international, randomised phase 3 study by the German Hodgkin Study Group. The Lancet Oncology 18(4):454–463. https://doi.org/10.1016/S1470-2045(17)30103-1
Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry D, Freeman GJ, Rodig SJ, Chapuy B, Ligon AH, Zhu L, Grosso JF, Kim SY, Timmerman JM, Shipp MA, Armand P (2015) PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372(4):311–319. https://doi.org/10.1056/NEJMoa1411087
Armand P, Shipp MA, Ribrag V, Michot JM, Zinzani PL, Kuruvilla J, Snyder ES, Ricart AD, Balakumaran A, Rose S, Moskowitz CH (2016) Programmed Death-1 blockade with pembrolizumab in patients with classical Hodgkin lymphoma after brentuximab vedotin failure. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. https://doi.org/10.1200/JCO.2016.67.3467
Bekoz H, Karadurmus N, Paydas S, Turker A, Toptas T, Firatli Tuglular T, Sonmez M, Gulbas Z, Tekgunduz E, Kaya AH, Ozbalak M, Tastemir N, Kaynar L, Yildirim R, Karadogan I, Arat M, Pepedil Tanrikulu F, Ozkocaman V, Abali H, Turgut M, Kurt Yuksel M, Ozcan M, Dogu MH, Kabukcu Hacioglu S, Barista I, Demirkaya M, Koseoglu FD, Toprak SK, Yilmaz M, Demirkurek HC, Demirkol O, Ferhanoglu B (2017) Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience. Ann Oncol 28(10):2496–2502. https://doi.org/10.1093/annonc/mdx341
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 32(27):3059–3068. https://doi.org/10.1200/JCO.2013.54.8800
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, Hoos A, Barrington SF, Armand P (2016) Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 128(21):2489–2496. https://doi.org/10.1182/blood-2016-05-718528
Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, Coiffier B, Fisher RI, Hagenbeek A, Zucca E, Rosen ST, Stroobants S, Lister TA, Hoppe RT, Dreyling M, Tobinai K, Vose JM, Connors JM, Federico M, Diehl V (2007) Revised response criteria for malignant lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 25(5):579–586. https://doi.org/10.1200/JCO.2006.09.2403
Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 (June 14, 2010).
Bair SM, Strelec L, Nagle SJ, Nasta SD, Landsburg DJ, Mato AR, Loren AW, Schuster SJ, Stadtmauer EA, Svoboda J (2017) Outcomes of patients with relapsed/refractory Hodgkin lymphoma progressing after autologous stem cell transplant in the current era of novel therapeutics: a retrospective analysis. Am J Hematol 92(9):879–884. https://doi.org/10.1002/ajh.24792
Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, Armand P, Fanale M, Ratanatharathorn V, Kuruvilla J, Cohen JB, Collins G, Savage KJ, Trneny M, Kato K, Farsaci B, Parker SM, Rodig S, Roemer MG, Ligon AH, Engert A (2016) Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol 17(9):1283–1294. https://doi.org/10.1016/S1470-2045(16)30167-X
Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, Radford J, Ribrag V, Molin D, Vassilakopoulos TP, Tomita A, von Tresckow B, Shipp MA, Zhang Y, Ricart AD, Balakumaran A, Moskowitz CH (2017) Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology:JCO2016721316. https://doi.org/10.1200/JCO.2016.72.1316
Armand P, Engert A, Younes A, Lee HJ, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, De Boer JP, Savage KJ, Kuruvilla J, Trněný M, Shipp MA, Sacchi M, Sumbul A, SM A (2018) Nivolumab for relapsed or refractory classical Hodgkin lymphoma (cHL) after autologous hematopoietic cell transplantation (auto-HCT): extended follow-up of the phase 2 single-arm CheckMate 205 study. Paper presented at the American Society of Hematology 60th Annual Meeting & Exposition 2018, San Diego, CA, December 1-4, 2018
Manson G, Mear JB, Herbaux C, Schiano JM, Casasnovas O, Stamatoullas A, Deau B, Schmitt A, Garnier G, Regny C, Bouabdallah K, Moles-Moreau MP, Ghesquieres H, Tempescul A, Dulery R, Nicolas-Virelizier E, Delmer A, Borel C, Chauchet A, Damotte D, Dercle L, Brice P, Houot R, Lysa (2019) Long-term efficacy of anti-PD1 therapy in Hodgkin lymphoma with and without allogenic stem cell transplantation. Eur J Cancer 115:47-56. https://doi.org/10.1016/j.ejca.2019.04.006
Martinez C, Carpio C, Heras I, Herranz ER, Buch J, Gutierrez A, Romero S, Ceberio I, Garcia-Garcia I, Izquierdo AR, Alonso R, Bargay J, Lekue CB, Javier N, de Haro ME, Palomera L, R G-S (2018) Nivolumab for heavily pretreated relapsed/refractory Hodgkin lymphoma: real-life experience in Spain (Spanish Group of Lymphoma and Bone Marrow Transplantation, GELTAMO). Paper presented at the American Society of Hematology 60th Annual Meeting & Exposition 2018, San Diego, CA, December 1-4, 2018
Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH, Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R (2012) Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol 30(18):2183–2189. https://doi.org/10.1200/JCO.2011.38.0410
Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, Timmerman JM, Collins GP, Ramchandren R, Cohen JB, De Boer JP, Kuruvilla J, Savage KJ, Trneny M, Shipp MA, Kato K, Sumbul A, Farsaci B, Ansell SM (2018) Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase ii CheckMate 205 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 36(14):1428–1439. https://doi.org/10.1200/JCO.2017.76.0793
Rossi C, Gilhodes J, Maerevoet M, Herbaux C, Morschhauser F, Brice P, Garciaz S, Borel C, Ysebaert L, Oberic L, Lazarovici J, Deau B, Dupuis J, Chauchet A, Abraham J, Bijou F, Stamatoullas-Bastard A, Malfuson JV, Golfier C, Laurent C, Pericart S, Traverse-Glehen A, Kanoun S, Filleron T, Casasnovas RO, Ghesquieres H (2018) Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: a series from Lysa centers. Am J Hematol. https://doi.org/10.1002/ajh.25154
Author information
Authors and Affiliations
Contributions
H.B., B.F., and M.Ozb. coordinated the study. B.F., H.B., S.P., and M.Ozb. wrote the manuscript. M.Ozb. performed the statistical evaluations. H.B., N.K., S.P., A.T., T.T., T.F.T., F.A., M.K.C., M.S., Z.G., N.D, L.K., R.Y., İ.K., M.A., N.A.A., V.O., M.T., M.K.Y., M.O., İ.K., S.K.H., I.B., M.D., G.S., S.K.T., M.Y., and B.F. contributed data. B.F. served as the principal investigator.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The NPP was organized after ethical approval, with the supervision of the Ministry of Health. The study was approved by the local ethical committee.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bekoz, H., Ozbalak, M., Karadurmus, N. et al. Nivolumab for relapsed or refractory Hodgkin lymphoma: real-life experience. Ann Hematol 99, 2565–2576 (2020). https://doi.org/10.1007/s00277-020-04077-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04077-4