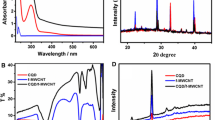
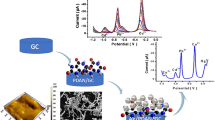
Abstract
Mercury as the 3rd most toxic, non-biodegradable, and carcinogenic pollutant can adversely affect the ecosystem and health of living species through its bioaccumulation within the nature that can affect the top consumer in the food chain; therefore, it is vital to sense/remove Hg2+ within/from aqueous media using practical approaches. To address this matter, we modified the glassy carbon electrode (GCE) with ultra-sensitive, interconnected, sulfurized, and porous nanostructure consisted of polyaniline-Fe3O4-silver diethyldithiocarbamate (PANi-F-S) to enhance the sensitivity, selectivity, and limit of detection (LOD) of the sensor. Obtained results showed that at optimum conditions (i.e., pH value of 7, deposition potential of − 0.8 V, and accumulation time of 120 s), for Hg2+ concentration ranging from 0.4 to 60 nM, the modified electrode showing linear relative coefficient of 0.9983, LOD of 0.051 nM, LOQ of 0.14 nM, and sensitivity of 1618.86 μA μM−1 cm−2 highlights superior sensitivity of the developed platform until picomolar level. Additionally, the modified electrode showed ideal repeatability, stability, reproducibility, and selectivity (by considering Zn2+, Cd2+ Pb2+, Cu2+, Ni2+, and Co2+ as metal interferences) and recovered more than 99% of the Hg2+ ions within non-biological (mineral, tap, and industrial waters) and biological (blood plasma sample) fluids.

Graphical abstract






Similar content being viewed by others
References
Gumpu MB, Sethuraman S, Krishnan UM, Rayappan JBB. A review on detection of heavy metal ions in water–an electrochemical approach. Sensors Actuators B Chem. 2015;213:515–33.
Ray PD, Yosim A, Fry RC. Incorporating epigenetic data into the risk assessment process for the toxic metals arsenic, cadmium, chromium, lead, and mercury: strategies and challenges. Front Genet. 2014;5:201.
ATSDR T ATSDR (Agency for Toxic Substances and Disease Registry). Prepared by Clement International Corp, under contract 205:88-0608.
Carocci A, Rovito N, Sinicropi MS, Genchi G. Mercury toxicity and neurodegenerative effects. In: Reviews of environmental contamination and toxicology: Springer. 2014;229:1–18.
Zhang Y, Zeng GM, Tang L, Chen J, Zhu Y, He XX, et al. Electrochemical sensor based on electrodeposited graphene-Au modified electrode and nanoAu carrier amplified signal strategy for attomolar mercury detection. Anal Chem. 2015;87(2):989–96.
Kidd K, Clayden M, Jardine T. Bioaccumulation and biomagnification of mercury through food webs. Hoboken: Environmental chemistry and toxicology of mercury Wiley; 2012. p. 455–99.
Chen G, Guo Z, Zeng G, Tang L. Fluorescent and colorimetric sensors for environmental mercury detection. Analyst. 2015;140(16):5400–43.
Hu J, Li J, Qi J, Chen J. Highly selective and effective mercury (II) fluorescent sensors. New J Chem. 2015;39(2):843–8.
Mandal P, Debbarma S, Saha A, Ruj B. Disposal problem of arsenic sludge generated during arsenic removal from drinking water. Procedia Environ Sci. 2016;35:943–9.
Li Y, Wang J, Luan Z, Liang Z. Arsenic removal from aqueous solution using ferrous based red mud sludge. J Hazard Mater. 2010;177(1–3):131–7.
Clarke ML, Harvey DG, Humphreys DJ.Veterinary toxicology. vol 2nd edition. London: Bailliere Tindall. 1981.
Bressler JP, Goldstein GW. Mechanisms of lead neurotoxicity. Biochem Pharmacol. 1991;41(4):479–84.
Rossi E. Low level environmental lead exposure–a continuing challenge. Clin Biochem Rev. 2008;29(2):63.
Ghosh A, Sen S, Sharma A, Talukder G. Effect of chlorophyllin on mercuric chloride-induced clastogenicity in mice. Food Chem Toxicol. 1991;29(11):777–9.
Solecki R, Hothorn L, Holzweissig M, Heinrich V. Computerised analysis of pathological findings in longterm trials with phenylmercuric acetate in rats. In: Recent developments in toxicology: trends, methods and problems: Springer. 1991;4:100–3.
Thuvander A, Sundberg J, Oskarsson A. Immunomodulating effects after perinatal exposure to methylmercury in mice. Toxicology. 1996;114(2):163–75.
Clarkson TW. Metal toxicity in the central nervous system. Environ Health Perspect. 1987;75:59–64.
Wright RO, Baccarelli A. Metals and neurotoxicology. J Nutr. 2007;137(12):2809–13.
Almeida P, Stearns LB. Political opportunities and local grassroots environmental movements: the case of Minamata. Soc Probl. 1998;45(1):37–60.
Hartwig A. Cadmium and cancer. In: Cadmium: from toxicity to essentiality: Springer. 2013;11:491–507.
Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem Res Toxicol. 2007;21(1):28–44.
Jeong ED, Won MS, Shim YB. Simultaneous determination of lead, copper, and mercury at a modified carbon paste eletrode containing humic acid. Electroanalysis. 1994;6(10):887–93.
Ugo P, Moretto LM, Mazzocchin GA. Voltammetric determination of trace mercury in chloride media at glassy carbon electrodes modified with polycationic ionomers. Anal Chim Acta. 1995;305(1–3):74–82.
Ugo P, Sperni L, Moretto LM. Ion-exchange voltammetry of trace mercury (II) at glassy carbon electrodes coated with a cationic polypyrrole derivative. Application to pore-waters analysis. Electroanalysis. 1997;9(15):1153–8.
Sharma SK, Kaur N, Singh J, Singh A, Raj P, Sankar S, et al. Salen decorated nanostructured ZnO chemosensor for the detection of mercuric ions (Hg2+). Sensors Actuators B Chem. 2016;232:712–21.
Li W, Li Y, Qian H-L, Zhao X, Yang C-X, Yan X-P. Fabrication of a covalent organic framework and its gold nanoparticle hybrids as stable mimetic peroxidase for sensitive and selective colorimetric detection of mercury in water samples. Talanta. 2019;204:224–8.
Mubarok AZ, Lin S-T, Mani V, Huang C-H, Huang S-T. Design of controlled multi-probe coupled assay via bioinspired signal amplification approach for mercury detection. RSC Adv. 2016;6(63):58485–92.
Jeromiyas N, Elaiyappillai E, Kumar AS, Huang S-T, Mani V. Bismuth nanoparticles decorated graphenated carbon nanotubes modified screen-printed electrode for mercury detection. J Taiwan Inst Chem Eng. 2019;95:466–74.
Manavalan S, Govindasamy M, Chen S-M, Rajaji U, Chen T-W, Ali MA, et al. Reduced graphene oxide supported raspberry-like SrWO4 for sensitive detection of catechol in green tea and drinking water samples. J Taiwan Inst Chem Eng. 2018;89:215–23.
Govindasamy M, Manavalan S, Chen S-M, Rajaji U, Chen T-W, Al-Hemaid FM, et al. Determination of neurotransmitter in biological and drug samples using gold nanorods decorated f-MWCNTs modified electrode. J Electrochem Soc. 2018;165(9):B370–7.
Govindasamy M, Rajaji U, Chen S-M, Kumaravel S, Chen T-W, Al-Hemaid FM, et al. Detection of pesticide residues (Fenitrothion) in fruit samples based on niobium carbide@ molybdenum nanocomposite: an electrocatalytic approach. Anal Chim Acta. 2018;1030:52–60.
Sharma VV, Tonelli D, Guadagnini L, Gazzano M. Copper-cobalt hexacyanoferrate modified glassy carbon electrode for an indirect electrochemical determination of mercury. Sensors Actuators B Chem. 2017;238:9–15.
Veerakumar P, Veeramani V, Chen S-M, Madhu R, Liu S-B. Palladium nanoparticle incorporated porous activated carbon: electrochemical detection of toxic metal ions. ACS Appl Mater Interfaces. 2016;8(2):1319–26.
Sakthinathan S, Tamizhdurai P, Ramesh A, Chiu T-W, Mangesh V, Veerarajan S, et al. Platinum incorporated mordenite zeolite modified glassy carbon electrode used for selective electrochemical detection of mercury ions. Microporous Mesoporous Mater. 2020;292:109770.
Shah A, Sultan S, Zahid A, Aftab S, Nisar J, Nayab S, et al. Highly sensitive and selective electrochemical sensor for the trace level detection of mercury and cadmium. Electrochim Acta. 2017;258:1397–403.
Rajabi HR, Roushani M, Shamsipur M. Development of a highly selective voltammetric sensor for nanomolar detection of mercury ions using glassy carbon electrode modified with a novel ion imprinted polymeric nanobeads and multi-wall carbon nanotubes. J Electroanal Chem. 2013;693:16–22.
Zhang P, Dong S, Gu G, Huang T. Simultaneous determination of Cd 2+, Pb 2+, Cu 2+ and Hg 2+ at a carbon paste electrode modified with ionic liquid-functionalized ordered mesoporous silica. Bull Kor Chem Soc. 2010;31(10):2949–54.
Jayadevimanoranjitham J, Narayanan SS. 2, 4, 6-Trimercaptotriazine incorporated gold nanoparticle modified electrode for anodic stripping voltammetric determination of Hg (II). Appl Surf Sci. 2018;448:444–54.
Zhou L, Xiong W, Liu S. Preparation of a gold electrode modified with Au–TiO 2 nanoparticles as an electrochemical sensor for the detection of mercury (II) ions. J Mater Sci. 2015;50(2):769–76.
Xing H, Xu J, Zhu X, Duan X, Lu L, Wang W, et al. Highly sensitive simultaneous determination of cadmium (II), lead (II), copper (II), and mercury (II) ions on N-doped graphene modified electrode. J Electroanal Chem. 2016;760:52–8.
Ordeig O, Banks CE, del Campo J, Muñoz FX, Compton RG. Trace detection of mercury (II) using gold ultra-microelectrode arrays. Electroanalysis Int J Devoted Fundam Pract Aspects Electroanalysis. 2006;18(6):573–8.
Sánchez-Calvo A, Fernández-Abedul MT, Blanco-López MC, Costa-García A. Based electrochemical transducer modified with nanomaterials for mercury determination in environmental waters. Sens Actuators B. 2019;290:87–92.
Augelli MA, Abarza Munoz RA, Richter EM, Gouveia Junior A, Angnes L. Chronopotentiometric stripping analysis using gold electrodes, an efficient technique for mercury quantification in natural waters. Electroanalysis Int J Devoted Fundam Pract Aspects Electroanalysis. 2005;17(9):755–61.
Shah A, Nisar A, Khan K, Nisar J, Niaz A, Ashiq MN, et al. Amino acid functionalized glassy carbon electrode for the simultaneous detection of thallium and mercuric ions. Electrochim Acta. 2019;321:134658.
Hashemi SA, Mousavi SM, Ramakrishna S. Effective removal of mercury, arsenic and lead from aqueous media using polyaniline-Fe3O4-silver diethyldithiocarbamate nanostructures. J Clean Prod. 2019;239:118023.
Van Erkelens P. Radiometric trace analysis of lead with diethyldithiocarbamate and 204T1. Anal Chim Acta. 1962;26:32–45.
Bobtelsky MM, Rafailoff R. A heterometric micro-determination of lead with sodium diethyldithiocarbamate. Anal Chim Acta. 1957;16:321–6.
Bobtelsky MM, Rafailoff R. Micro-heterometric determination of bismuth with sodium diethyldithiocarbamate. Anal Chim Acta. 1957;16:488–92.
Merry RH, Zarcinas B. Spectrophotometric determination of arsenic and antimony by the silver diethyldithiocarbamate method. Analyst. 1980;105(1251):558–63.
Alvarez-Coque MG, Camañas RV, Vaya MM, Ramos GR, Fernandez CM. Spectrophotometric determination of mercury (II) and silver (I) with copper (II) and diethyldithiocarbamate in the presence of triton X-100. Talanta. 1986;33(8):697–9.
Bobtelsky M, Rafailoff R. A precise, direct, heterometric micro-determination of mercury with diethyldithiocarbamate in excesses of metals ethylenediaminetetraacetate solutions. Anal Chim Acta. 1956;14:339–43.
Bobtelsky M, Rafailoff R. A precise direct heterometric determination of traces of copper with diethyldithiocarbamate in excesses of metals. Anal Chim Acta. 1956;14:558–67.
Urbowicz G, Krysiak A, Lulek J. Application of the silver diethyldithiocarbamate spectrophotometric method to determine total arsenic in some mineral waters. Bromatol Chem Toksykol. 2005;38(3):247.
Mousavi M, Hashemi A, Arjmand O, Amani AM, Babapoor A, Fateh MA, et al. Erythrosine adsorption from aqueous solution via decorated graphene oxide with magnetic iron oxide nano particles: kinetic and equilibrium studies. Acta Chim Slov. 2018;65(4):882–94.
Mousavi SM, Hashemi SA, Amani AM, Esmaeili H, Ghasemi Y, Babapoor A, et al. Pb (II) removal from synthetic wastewater using Kombucha Scoby and graphene oxide/Fe3O4. Physical Chemistry Research. 2018;6(4):759–71.
Mousavi SM, Hashemi SA, Esmaeili H, Amani AM, Mojoudi F. Synthesis of Fe3O4 nanoparticles modified by oak shell for treatment of wastewater containing Ni (II). Acta Chim Slov. 2018;65(3):750–6.
Bahrani S, Razmi Z, Ghaedi M, Asfaram A, Javadian H. Ultrasound-accelerated synthesis of gold nanoparticles modified choline chloride functionalized graphene oxide as a novel sensitive bioelectrochemical sensor: optimized meloxicam detection using CCD-RSM design and application for human plasma sample. Ultrason Sonochem. 2018;42:776–86. https://doi.org/10.1016/j.ultsonch.2017.12.042.
Xie G, Xi P, Liu H, Chen F, Huang L, Shi Y, et al. A facile chemical method to produce superparamagnetic graphene oxide–Fe 3 O 4 hybrid composite and its application in the removal of dyes from aqueous solution. J Mater Chem. 2012;22(3):1033–9.
Ai L, Zhang C, Chen Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J Hazard Mater. 2011;192(3):1515–24.
Cao H, Zhou X, Zhang Y, Chen L, Liu Z. Microspherical polyaniline/graphene nanocomposites for high performance supercapacitors. J Power Sources. 2013;243:715–20.
Wang L, Chen L, Yan B, Wang C, Zhu F, Jiang X, et al. In situ preparation of SnO 2@ polyaniline nanocomposites and their synergetic structure for high-performance supercapacitors. J Mater Chem A. 2014;2(22):8334–41.
Pang H, Huang C, Chen J, Liu B, Kuang Y, Zhang X. Preparation of polyaniline–tin dioxide composites and their application in methanol electro-oxidation. J Solid State Electrochem. 2010;14(2):169.
Xu XH, Ren GL, Cheng J, Liu Q, Li DG, Chen Q. Self-assembly of polyaniline-grafted chitosan/glucose oxidase nanolayered films for electrochemical biosensor applications. J Mater Sci. 2006;41(15):4974–7.
Han MG, Cho SK, Oh SG, Im SS. Preparation and characterization of polyaniline nanoparticles synthesized from DBSA micellar solution. Synth Met. 2002;126(1):53–60.
Jafari MJ. Application of vibrational spectroscopy in organic electronics: 2017-11-17, Planck, F-House, Campus Valla, Linköping, 10:15 (English): Linköping University Electronic Press. 2017, 61
Zacca M-J, Laurencin D, Richeter S, Clément S, Mehdi A. New layered polythiophene-silica composite through the self-assembly and polymerization of thiophene-based silylated molecular precursors. Molecules. 2018;23(10):2510.
Silva A, Ugucion J, Correa S, Ardisson J, Macedo W, Silva J, et al. Synthesis and characterization of nanocomposites consisting of polyaniline, chitosan and tin dioxide. Mater Chem Phys. 2018;216:402–412.
Yu T, Zhu P, Xiong Y, Chen H, Kang S, Luo H, et al. Synthesis of microspherical polyaniline/graphene composites and their application in supercapacitors. Electrochim Acta. 2016;222:12–9.
Palsaniya S, Nemade HB, Dasmahapatra AK. Synthesis of polyaniline/graphene/MoS2 nanocomposite for high performance supercapacitor electrode. Polymer. 2018;150:150–8.
Kumar S, Lei Y, Alshareef NH, Quevedo-Lopez MA, Salama KN. Biofunctionalized two-dimensional Ti3C2 MXenes for ultrasensitive detection of cancer biomarker. Biosens Bioelectron. 2018;121:243–9. https://doi.org/10.1016/j.bios.2018.08.076.
Bagheri H, Afkhami A, Khoshsafar H, Rezaei M, Shirzadmehr A. Simultaneous electrochemical determination of heavy metals using a triphenylphosphine/MWCNTs composite carbon ionic liquid electrode. Sensors Actuators B Chem. 2013;186:451–60. https://doi.org/10.1016/j.snb.2013.06.051.
Wang N, Lin M, Dai H, Ma H. Functionalized gold nanoparticles/reduced graphene oxide nanocomposites for ultrasensitive electrochemical sensing of mercury ions based on thymine-mercury-thymine structure. Biosens Bioelectron. 2016;79:320–6. https://doi.org/10.1016/j.bios.2015.12.056.
Liu W, Wu X, Li X. Gold nanorods on three-dimensional nickel foam: a non-enzymatic glucose sensor with enhanced electro-catalytic performance. RSC Adv. 2017;7(58):36744–9. https://doi.org/10.1039/c7ra06909j.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The studies have been performed in accordance with the ethical standards and approved through following the protocols of the ethical committee. The human blood plasma sample is provided from the stock of Fars Blood Transfusion Center (Shiraz, Fars, Iran) that was obtained from a healthy volunteer with full consent of donor. The reference and LOT of the supplied human blood plasma sample are MDE650Q and 11275071CM, respectively.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 234 kb)
Rights and permissions
About this article
Cite this article
Hashemi, S.A., Mousavi, S.M., Bahrani, S. et al. Picomolar-level detection of mercury within non-biological/biological aqueous media using ultra-sensitive polyaniline-Fe3O4-silver diethyldithiocarbamate nanostructure. Anal Bioanal Chem 412, 5353–5365 (2020). https://doi.org/10.1007/s00216-020-02750-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02750-1