Abstract
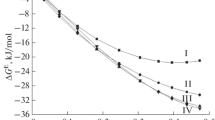
The thermodynamic properties of the Sm2O3–Y2O3–HfO2 system at 2500 K were modeled based on the generalized lattice theory of associated solutions using experimental data obtained by high-temperature mass spectrometry. A comparison was made between the results of the calculations of the thermodynamic properties of the Sm2O3–Y2O3–HfO2 system using two independent approaches to modeling based on this theory: from the experimental data on the properties of the ternary system and from the data on its boundary binary systems Sm2O3–Y2O3, Sm2O3–HfO2, and Y2O3–HfO2. It was shown that the chemical potentials of the components that were calculated in the studied ternary system using the above approaches much better fit the experimental data in the former case.



Similar content being viewed by others
REFERENCES
J. A. Barker, J. Chem. Phys. 20, 1526 (1952).
J. A. Barker and F. Smith, J. Chem. Phys. 22, 375 (1954). https://doi.org/10.1063/1.1740077
L. M. Kurtynina, N. A. Smirnova, and P. F. Andrukovich, Khim. Termodin. Rastvorov, No. 2, 43 (1968).
M. V. Alekseeva, K. Zyunel’, E. M. Piotrovskaya, et al., Vestn. Leningr. Univ., No. 11, 43 (1985).
J. R. Goats, J. B. Ott, R. L. Snow, et al., J. Chem. Thermodyn. 12, 447 (1980). https://doi.org/10.1016/0021-9614(80)90058-0
M. M. Shul’ts, G. G. Ivanov, V. L. Stolyarova, et al., Fiz. Khim. Stekla 12, 285 (1986).
M. M. Shul’ts, G. G. Ivanov, V. L. Stolyarova, et al., Fiz. Khim. Stekla 12, 385 (1986).
M. M. Shul’ts, G. G. Ivanov, and V. L. Stolyarova, Dokl. Akad. Nauk SSSR 292 (5), 1198 (1987).
V. L. Stolyarova, G. G. Ivanov, and M. M. Shul’ts, Dokl. Akad. Nauk SSSR 305 (2), 383 (1989).
V. L. Stolyarova, Russ. Chem. Rev. 85, 60 (2016). https://doi.org/10.1070/RCR4549
V. L. Stolyarova, CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 64, 258 (2019). https://doi.org/10.1016/j.calphad.2018.12.013
V. L. Stolyarova, S. I. Lopatin, S. M. Shugurov, et al., Russ. J. Gen. Chem. 80, 689 (2010). https://doi.org/10.1134/S1070363210040018
V. L. Stolyarova, S. I. Lopatin, S. M. Shugurov, et al., Russ. J. Gen. Chem. 80, 2405 (2010). https://doi.org/10.1134/S1070363210120029
V. L. Stolyarova, S. I. Lopatin, and A. L. Shilov, Russ. J. Gen. Chem. 79, 1778 (2009). https://doi.org/10.1134/S1070363209090035
V. L. Stolyarova and A. L. Shilov, J. Non-Cryst. Solids 366, 6 (2013). https://doi.org/10.1016/j.jnoncrysol.2013.01.036
V. L. Stolyarova, A. L. Shilov, S. I. Lopatin, et al., Rapid Comm. Mass. Spectrom. 28, 801 (2014). https://doi.org/10.1002/rcm.6842
V. L. Stolyarova, S. I. Lopatin, S. M. Shugurov, et al., in Proceedings of the XIV Russian Conference with International Participation on Thermophysical Properties of Substances (RKTS-14), Kazan, Russia, October 15–17, 2014 (KNITU, Kazan, 2014).
V. V. Golubkov, P. A. Onushchenko, and V. L. Stolyarova, Glass Phys. Chem. 41, 247 (2015). https://doi.org/10.1134/S1087659615030074
V. V. Golubkov, P. A. Onushchenko, and V. L. Stolyarova, Glass Phys. Chem. 39, 624 (2013). https://doi.org/10.1134/S1087659613060059
V. V. Golubkov and V. L. Stolyarova, Glass Phys. Chem. 37, 252 (2011). https://doi.org/10.1134/S1087659611030047
V. V. Golubkov and V. L. Stolyarova, Glass Phys. Chem. 32, 287 (2006). https://doi.org/10.1134/S1087659606030059
A. L. Shilov, S. V. Stolyar, V. L. Stolyarova, et al., Glass Technol.: Eur. J. Glass Sci. Technol. A 60, 105 (2019). https://doi.org/10.13036/17533546.60.4.016
A. L. Shilov, S. I. Lopatin, V. L. Stolyarova, et al., JALCOM 791, 1207 (2019). https://doi.org/10.1016/j.jallcom.2019.03.182
V. A. Vorozhtcov, A. L. Shilov, and V. L. Stolyarova, Russ. J. Gen. Chem. 89, 475 (2019). https://doi.org/10.1134/S1070363219030186
A. L. Shilov, V. L. Stolyarova, V. A. Vorozhtcov, et al., CALPHAD: Comput. Coupling Phase Diagrams Thermochem. 65, 165 (2019). https://doi.org/10.1016/j.calphad.2019.03.001
E. N. Kablov, V. L. Stolyarova, S. I. Lopatin, et al., Rapid Comm. Mass. Spectrom. 31, 538 (2017). https://doi.org/10.1002/rcm.7809
E. N. Kablov, V. L. Stolyarova, S. I. Lopatin, et al., Rapid Comm. Mass. Spectrom. 31, 1137 (2017). https://doi.org/10.1002/rcm.7892
E. N. Kablov, V. L. Stolyarova, V. A. Vorozhtcov, et al., Rapid Comm. Mass. Spectrom. 32, 686 (2018). https://doi.org/10.1002/rcm.8081
A. N. Belov and G. A. Semenov, Zh. Fiz. Khim. 59, 589 (1985).
Funding
This work was supported by the Russian Foundation for Basic Research (project no. 19-03-00721).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Glyanchenko
Rights and permissions
About this article
Cite this article
Shilov, A.L., Stolyarova, V.L., Vorozhtcov, V.A. et al. Optimization of the Thermodynamic Properties of the Sm2O3–Y2O3–HfO2 System at High Temperatures by the Barker Method. Russ. J. Inorg. Chem. 65, 773–780 (2020). https://doi.org/10.1134/S0036023620050216
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620050216