Abstract
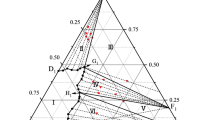
A set of Pitzer parameters was obtained to accurately describe the thermodynamic properties of solutions in the H2O–UO2(NO3)2–Th(NO3)4–HNO3 system at 25°С and represent the thermodynamic properties of liquid phases in the H2O–Th(NO3)4–HNO3 subsystem in the temperature range 25–50°С. Solubility products were calculated for crystal hydrates Th(NO3)4 · 6H2O and UO2(NO3)2 · 3H2O to predict the solubilities of these compounds in those solutions over a wide concentration range.



Similar content being viewed by others
REFERENCES
R. Nascimento, J. Moreira, and L. Cardoso, Rev. Caatinga 30, 213 (2017). https://doi.org/10.1590/1983-21252017v30n123rc
A. S. Maliutin, N. A. Kovalenko, and I. A. Uspenskaya, Moscow Univ. Chem. Bull. 75, 65 (2020). https://doi.org/10.3103/S0027131420020091
K. S. Pitzer, J. Phys. Chem. 77, 268 (1973). https://doi.org/10.1021/j100621a026
K. S. Pitzer, P. Wang, and J. Rard, J. Solution Chem. 28, 265 (1999). https://doi.org/10.1023/A:1022695525943
A. L. Voskov, N. A. Kovalenko, and I. B. Kutsenok, Russ. J. Phys. Chem. A 93, 1849 (2019). https://doi.org/10.1134/S0036024419100327
R. A. Robinson and B. J. Levien, Trans. Proc. R. Soc. New Zeal. 76, 295 (1946).
R. J. Lemire, N. H. Sagert, and D. W. P. Lau, J. Chem. Eng. Data 29, 329 (1984). https://doi.org/10.1021/je00037a031
A. Apelblat, D. Azoulay, and A. Sahar, J. Chem. Soc., Faraday Trans. 1: Phys. Chem. Condens. Phases 69, 1624 (1973). https://doi.org/10.1039/f19736901624
W. L. Marshall, J. S. Gill, and C. H. Secoy, J. Am. Chem. Soc. 73, 4991 (1951). https://doi.org/10.1021/ja01154a531
A. Apelblat, D. Azoulay, and A. Sahar, J. Chem. Soc., Faraday Trans. 1: Phys. Chem. Condens. Phases 69, 1618 (1973). https://doi.org/10.1039/f19736901618
R. J. Lemire and C. P. Brown, J. Solution Chem. 11, 203 (1982). https://doi.org/10.1007/BF00667602
R. J. Lemire, C. P. Brown, and A. B. Campbell, J. Chem. Eng. Data 30, 421 (1985). https://doi.org/10.1021/je00042a015
J. R. Ferraro, L. I. Katzin, and G. Gibson, J. Am. Chem. Soc. 76, 909 (1954). https://doi.org/10.1021/ja01632a083
V. I. Volk, A. Yu. Vakhrushin, and S. L. Mamaev, Radiochemistry 41, 222 (1999).
E. Lange and W. Miederer, Ber. Bunsen-Ges. Phys. Chem. 61, 407 (1957). https://doi.org/10.1002/bbpc.19570610317
A. Apelblat and A. Sahar, J. Chem. Soc., Faraday Trans. 1: Phys. Chem. Condens. Phases 71, 1667 (1975). https://doi.org/10.1039/f19757101667
V. I. Volk, A. Yu. Vakhrushin, and S. L. Mamaev, J. Radioanal. Nucl. Chem. 243, 703 (2000). https://doi.org/10.1023/A:1006764417407
K. S. Pitzer and G. Mayorga, J. Phys. Chem. 77, 2300 (1973). https://doi.org/10.1021/j100638a009
H. T. Kim and W. J. Frederick, J. Chem. Eng. Data 33, 177 (1988). https://doi.org/10.1021/je00052a035
M. Simoes, K. Hughes, and D. Ingham, J. Chem. Eng. Data 61, 2536 (2016). https://doi.org/10.1021/acs.jced.6b00236
Funding
This study was supported by the Russian Foundation for Basic Research (project no. 18-29-24167).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Fedorova
Rights and permissions
About this article
Cite this article
Malyutin, A.S., Kovalenko, N.A. & Uspenskaya, I.A. Thermodynamic Properties of Phases and Phase Equilibria in the H2O–HNO3–UO2(NO3)2–Th(NO3)4 System. Russ. J. Inorg. Chem. 65, 781–786 (2020). https://doi.org/10.1134/S0036023620050149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036023620050149