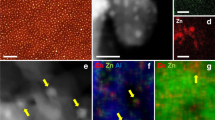
Abstract
The selective catalytic oxidation of methanol to methyl formate (MF) has been considered as an attractive route to produce MF, an important precursor in the chemical industry. Although supported Pd nanoparticles are active for the selective oxidation of methanol to MF, the relatively low MF selectivity and yield remains an unsolved issue. Herein, we show that adding a small amount of Pd into Cu forming PdCu single atom alloy (SAA) catalysts can catalyze the oxidation of methanol to MF selectively at low temperatures, with a high catalyst stability. The atomic dispersion of Pd atoms in the Cu matrix was confirmed by various in-situ characterization techniques. The activity and selectivity of PdCu SAA catalysts were compared to monometallic Cu and Pd catalysts, in a flow reactor at similar conditions. This work may guide the design of new SAA based catalysts for selective oxidation reactions.
Graphic Abstract









Similar content being viewed by others
References
Ullmann F, Bohnet M (2009) Ullmann's encyclopedia of industrial chemistry. Wiley, New York
Wittstock A, Zielasek V, Biener J, Friend CM, Baumer M (2010) Nanoporous gold catalysts for selective gas-phase oxidative coupling of methanol at low temperature. Science 327:319–322
Li W, Liu H, Iglesia E (2006) Structures and properties of zirconia-supported ruthenium oxide catalysts for the selective oxidation of methanol to methyl formate. J Phys Chem B 110:23337–23342
Lee JS, Kim JC, Kim YG (1990) Methyl formate as a new building block in C1 chemistry. Appl Catal 57:1–30
Gérard E, Götz H, Pellegrini S, Castanet Y, Mortreux A (1998) Epoxide–tertiary amine combinations as efficient catalysts for methanol carbonylation into methyl formate in the presence of carbon dioxide. Appl Catal A 170:297–306
He L, Liu H, Xiao C-X, Kou Y (2008) Liquid-phase synthesis of methyl formate via heterogeneous carbonylation of methanol over a soluble copper nanocluster catalyst. Green Chem 10:619
Whiting GT, Kondrat SA, Hammond C, Dimitratos N, He Q, Morgan DJ, Dummer NF, Bartley JK, Kiely CJ, Taylor SH, Hutchings GJ (2014) Methyl formate formation from methanol oxidation using supported gold–palladium nanoparticles. Acs Catal 5:637–644
Cao S, Yang M, Elnabawy AO, Trimpalis A, Li S, Wang C, Göltl F, Chen Z, Liu J, Shan J, Li M, Haas T, Chapman KW, Lee S, Allard LF, Mavrikakis M, Flytzani-Stephanopoulos M (2019) Single-atom gold oxo-clusters prepared in alkaline solutions catalyse the heterogeneous methanol self-coupling reactions. Nat Chem 11:1098–1105
Li C, Yang X, Gao G, Li Y, Zhang W, Chen X, Su H, Wang S, Wang Z (2019) Copper on the inner surface of mesoporous TiO2 hollow spheres: a highly selective photocatalyst for partial oxidation of methanol to methyl formate. Catal Sci Technol 9:6240–6252
Zhang Q, Zhu C, Yang G, Sun Y, Wang D, Liu J (2019) High-performance microstructured Au–Ag bimetallic catalyst for oxidative coupling of methanol to methyl formate. Catal Commun 129:105741
Yu H, Zeng K, Fu X, Zhang Y, Peng F, Wang H, Yang J (2008) RuO2·xH2O Supported on carbon nanotubes as a highly active catalyst for methanol oxidation. J Phys Chem C 112:11875–11880
Huang H, Li W, Liu H (2012) Effect of treatment temperature on structures and properties of zirconia-supported ruthenium oxide catalysts for selective oxidation of methanol to methyl formate. Catal Today 183:58–64
Liu Z, Zhang R, Wang S, Li N, Sima R, Liu G, Wu P, Zeng G, Li S, Sun Y (2016) Highly efficient and stable vanadia–titania–sulfate catalysts for methanol oxidation to methyl formate: synthesis and mechanistic study. J Phys Chem C 120:6591–6600
Liu J, Zhan E, Cai W, Li J, Shen W (2007) Methanol selective oxidation to methyl formate over ReOx/CeO2 catalysts. Catal Lett 120:274–280
Li N, Wang S, Sun Y, Li S (2017) First principles studies on the selectivity of dimethoxymethane and methyl formate in methanol oxidation over V2O5/TiO2-based catalysts. Phys Chem Chem Phys 19:19393–19406
Kaichev VV, Popova GY, Chesalov YA, Saraev AA, Zemlyanov DY, Beloshapkin SA, Knop-Gericke A, Schlögl R, Andrushkevich TV, Bukhtiyarov VI (2014) Selective oxidation of methanol to form dimethoxymethane and methyl formate over a monolayer V2O5/TiO2 catalyst. J Catal 311:59–70
Wojcieszak R, Karelovic A, Gaigneaux EM, Ruiz P (2014) Oxidation of methanol to methyl formate over supported Pd nanoparticles: insights into the reaction mechanism at low temperature. Catal Sci Technol 4:3298–3305
Yang Z, Li J, Yang X, Wu Y (2005) Catalytic oxidation of methanol to methyl formate over silver? a new purpose of a traditional catalysis system. Catal Lett 100:205–211
Han C, Yang X, Gao G, Wang J, Lu H, Liu J, Tong M, Liang X (2014) Selective oxidation of methanol to methyl formate on catalysts of Au–Ag alloy nanoparticles supported on titania under UV irradiation. Green Chem 16:3603–3615
Wang R, Wu Z, Chen C, Qin Z, Zhu H, Wang G, Wang H, Wu C, Dong W, Fan W, Wang J (2013) Graphene-supported Au–Pd bimetallic nanoparticles with excellent catalytic performance in selective oxidation of methanol to methyl formate. Chem Commun 49:8250
Wojcieszak R, Gaigneaux EM, Ruiz P (2013) Direct methyl formate formation from methanol over supported palladium nanoparticles at low temperature. Chemcatchem 5:339–348
Wu J-B, Shi R-P, Qin Z-F, Liu H, Li Z-K, Zhu H-Q, Zhao Y-X, Wang J-G (2019) Selective oxidation of methanol to methyl formate over bimetallic Au–Pd nanoparticles supported on SiO2. J Fuel Chem Technol 47:780–790
Wang L-C, Personick ML, Karakalos S, Fushimi R, Friend CM, Madix RJ (2016) Active sites for methanol partial oxidation on nanoporous gold catalysts. J Catal 344:778–783
Xu B, Siler CGF, Madix RJ, Friend CM (2014) Ag/Au mixed sites promote oxidative coupling of methanol on the alloy surface. Chem Eur J 20:4646–4652
Flytzani-Stephanopoulos M, Gates BC (2012) Atomically dispersed supported metal catalysts. Ann Rev Chem Biomol Eng 3:545–574
Yang XF, Wang AQ, Qiao BT, Li J, Liu JY, Zhang T (2013) Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc Chem Res 46:1740–1748
Liu JY (2017) Catalysis by Supported Single Metal Atoms. Acs Catal 7:34–59
Kyriakou G, Boucher MB, Jewell AD, Lewis EA, Lawton TJ, Baber AE, Tierney HL, Flytzani-Stephanopoulos M, Sykes ECH (2012) Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335:1209–1212
Boucher MB, Zugic B, Cladaras G, Kammert J, Marcinkowski MD, Lawton TJ, Sykes ECH, Flytzani-Stephanopoulos M (2013) Single atom alloy surface analogs in Pd0.18Cu15 nanoparticles for selective hydrogenation reactions. Phys Chem Chem Phys 15:12187–12196
Lucci FR, Liu JL, Marcinkowski MD, Yang M, Allard LF, Flytzani-Stephanopoulos M, Sykes ECH (2015) Selective hydrogenation of 1,3-butadiene on platinum-copper alloys at the single-atom limit. Nat Commun 6:1–8
Liu JL, Lucci FR, Yang M, Lee S, Marcinkowski MD, Therrien AJ, Williams CT, Sykes ECH, Flytzani-Stephanopoulos M (2016) Tackling CO poisoning with single-atom alloy catalysts. J Am Chem Soc 138:6396–6399
Shan J, Lucci FR, Liu J, El-Soda M, Marcinkowski MD, Allard LF, Sykes ECH, Flytzani-Stephanopoulos M (2016) Water co-catalyzed selective dehydrogenation of methanol to formaldehyde and hydrogen. Surf Sci 650:121–129
Liu J, Shan J, Lucci FR, Cao S, Sykes ECH, Flytzani-Stephanopoulos M (2017) Palladium–gold single atom alloy catalysts for liquid phase selective hydrogenation of 1-hexyne. Catal Sci Technol 7:4276–4284
Shan J, Janvelyan N, Li H, Liu J, Egle TM, Ye J, Biener MM, Biener J, Friend CM, Flytzani-Stephanopoulos M (2017) Selective non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen on highly dilute NiCu alloys. Appl Catal B 205:541–550
Shan J, Liu J, Li M, Lustig S, Lee S, Flytzani-Stephanopoulos M (2018) NiCu single atom alloys catalyze the C H bond activation in the selective non- oxidative ethanol dehydrogenation reaction. Appl Catal B 226:534–543
Marcinkowski MD, Darby MT, Liu J, Wimble JM, Lucci FR, Lee S, Michaelides A, Flytzani-Stephanopoulos M, Stamatakis M, Sykes ECH (2018) Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C–H activation. Nat Chem 10:325–332
Giannakakis G, Flytzani-Stephanopoulos M, Sykes ECH (2018) Single-atom alloys as a reductionist approach to the rational design of heterogeneous catalysts. Acc Chem Res 52:237–247
Podborska A, Wojnicki M (2017) Spectroscopic and theoretical analysis of Pd2+−Cl−−H2O system. J Mol Struct 1128:117–122
Dhas NA, Raj CP, Gedanken A (1998) Synthesis, characterization, and properties of metallic copper nanoparticles. Chem Mater 10:1446–1452
Subramanian ND, Kumar CSSR, Watanabe K, Fischer P, Tanaka R, Spivey JJ (2012) A DRIFTS study of CO adsorption and hydrogenation on Cu-based core-shell nanoparticles. Catal Sci Technol 2:621–631
Shan S, Petkov V, Prasai B, Wu J, Joseph P, Skeete Z, Kim E, Mott D, Malis O, Luo J, Zhong C-J (2015) Catalytic activity of bimetallic catalysts highly sensitive to the atomic composition and phase structure at the nanoscale. Nanoscale 7:18936–18948
Sarkar C, Koley P, Shown I, Lee J, Liao Y-F, An K, Tardio J, Nakka L, Chen K-H, Mondal J (2019) Integration of interfacial and alloy effects to modulate catalytic performance of metal–organic-framework-derived Cu–Pd nanocrystals toward hydrogenolysis of 5-hydroxymethylfurfural. ACS Sustain Chem Eng 7:10349–10362
Olekszyszen DN, Albuquerque BL, Silva DD, Tripodi GL, de Oliveira DC, Domingos JB (2020) Domingos, Core–shell PdCu bimetallic colloidal nanoparticles in Sonogashira cross-coupling reaction: mechanistic insights into the catalyst mode of action. Nanoscale 12:1171–1179
Lucci FR, Zhang L, Thuening T, Uhlman MB, Schilling AC, Henkelman G, Charles E, Sykes H (2018) The effect of single pd atoms on the energetics of recombinative O2 desorption from Au(111). Surf Sci 677:296–300
Trimpalis A, Giannakakis G, Cao S, Flytzani-Stephanopoulos M (2019) NiAu single atom alloys for the selective oxidation of methacrolein with methanol to methyl methacrylate. Catal Today. https://doi.org/10.1016/j.cattod.2019.04.021
Darby MT, Stamatakis M, Michaelides A, Sykes ECH (2018) Lonely atoms with special gifts: breaking linear scaling relationships in heterogeneous catalysis with single-atom alloys. J Phys Chem Lett 9:5636–5646
Acknowledgements
This work was supported as part of the Integrated Mesoscale Architectures for Sustainable Catalysis, an Energy Frontier Research Center funded by the U.S. Department of Energy, Office of Science, Basic Energy Sciences under award #DESC0012573. The XAS research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science, User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest.
Research Involving Human Participants and/or Animal Rights
There were no human or animal subjects involved in this research.
Informed Consent
The authors state that the manuscript has not been published or submitted to any other journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shan, J., Giannakakis, G., Liu, J. et al. PdCu Single Atom Alloys for the Selective Oxidation of Methanol to Methyl Formate at Low Temperatures. Top Catal 63, 618–627 (2020). https://doi.org/10.1007/s11244-020-01288-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01288-x