Abstract
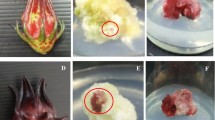
The present study, for the first time, assessed the influence of light intensity on morphogenesis of callus cultures and accumulation of β-sitosterol, a key phytosterol compound in date palm. High frequencies of calli with maximum biomass were induced from shoot tip explants in Murashige and Skoog basal medium supplemented with 10 mg/l 2,4 dichlorophenoxyacetic acid + 3 mg/l 2-isopentyladenine. Such proliferating callus cultures of date palm cv. Hayani were incubated in specified medium under different light intensities [0, 14, and 42 µmol/m2/s photosynthetic photon flux density (PPFD)]. The collected data exhibited an increase in callus volume, globularization, newly differentiated somatic embryos, degree of browning, and β-sitosterol contents of callus cultures. Various effects of different light intensities on the above-mentioned traits were recorded. Morphogenesis of callus under dark condition (0 µmol/m2/s PPFD) resulted in a higher degree of globularization and produced maximum number of newly differentiated somatic embryos. Whereas, under gradually increasing light intensities (14 and 42 µmol/m2/s PPFD), a surge in the accumulation of β-sitosterol content in the proliferating callus was observed. Thus, from the present study, it can be deduced that a proper application of suitable light intensity on proliferating callus has beneficial influence on morphogenesis and a higher production of β-sitosterol in date palm.




Similar content being viewed by others
Availability of Data and Material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2,4-D:
-
2,4 dichlorophenoxyacetic acid
- 2iP:
-
2-isopentyladenine
- ANOVA:
-
Analysis of variance
- MS:
-
Murashige and Skoog
- NAA:
-
α-napthalene acetic acid
- NOA:
-
Naphthoxyacetic acid
- PPFD:
-
Photosynthetic photon flux density
References
Abd El-Kadder, E.M., I.I. Lashin, M.S. Aref, E.A. Hussian, and E.A. Ewais. 2014. Physical elicitation of (Dillenia indica) callus for production of secondary metabolites. New York Science Journal 7: 48–57.
Aerts, R.J., and V. De Luca. 1992. Phytochrome is involved in the light-regulation of vindoline biosynthesis in Catharanthus. Plant Physiology 100: 1029–1032.
Afshari, R., R. Angoshtari, and S. Kalantari. 2011. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (‘Brassica napus L’.) genotypes. Plant Omics 4: 60–67.
Ahmad, N., A. Rab, and N. Ahmad. 2016. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of (Stevia rebaudiana) (Bert). Journal of Photochemistry and Photobiology B: Biology 154: 51–56.
Al-Alawi, R., J. Al-Mashiqri, J. Al-Nadabi, B. Al-Shihi, and Y. Baq. 2017. Date palm tree (Phoenix dactylifera L.): Natural products and therapeutic options. Frontiers in Plant Science 8: 845.
Al-Mssallem, M.Q., R.M. Alqurashi, and J.M. Al-Khayri. 2019. Bioactive compounds of date palm (Phoenix dactylifera L.). In Bioactive compounds in underutilized fruits and nuts, Reference series in phytochemistry, ed. H.N. Murthy and V.A. Bapat, 1–15. Switzerland: Springer.
Alvarenga, I.C.A., F.V. Pacheco, S.T. Silva, S.K.V. Bertolucci, and J.E.B.P. Pinto. 2015. In vitro culture of Achillea millefolium L: quality and intensity of light on growth and production of volatiles. Plant Cell, Tissue and Organ Culture 122: 299–308.
Bekheet, S.A. 2013. Direct organogenesis of date palm (Phoenix dactylifera L.) for propagation of true-to-type plants. Scientia Agriculturae 4: 85–92.
Billore, V., L. Khatediya, and M. Jain. 2016. Sink-source system of in vitro suspension culture of (Celastrus paniculatus) under regulation of monochromatic lights. Plant Tissue Culture and Biotechnology 26: 175–185.
Bin Sayeed, M.S., S.M.R. Karim, T. Sharmin, and M.M. Morshed. 2016. Critical analysis on characterization, systemic effect, and therapeutic potential of beta-sitosterol: a plant-derived orphan phytosterol. Medicines 3: 29.
Chan, L.K., S.S. Koay, P.L. Boey, and A. Bhatt. 2010. Effects of abiotic stress on biomass and anthocyanin production in cell cultures of Melastoma malabathricum. Biological Research 43: 127–135.
De-Eknamkul, W., and B. Potduang. 2003. Biosynthesis of β-sitosterol and stigmasterol in Croton sublyratus proceeds via a mixed origin of isoprene units. Phytochemistry 62: 389–398.
Dewick, P.M. 2002. Medicinal natural products: a biosynthetic approach. New Jersey: Wiley.
Duncan, D.B. 1955. Multiple range and multiple F tests. Biometrics 11: 1–42.
El-Dawayati, M.M., H.S. Ghazzawy, and M. Munir. 2018. Somatic embryogenesis enhancement of date palm cultivar Sewi using different types of polyamines and glutamine amino acid concentration under in vitro solid and liquid media conditions. International Journal of Biosciences 12: 149–159.
El-Hadrami, A., F. Daayf, and I. El-Hadrami. 2011. Secondary metabolites of date palm. In Date palm biotechnology, ed. S.M. Jain, J.M. Al-Khayri, and D. Johnson, 653–674. Dordrecht: Springer.
El-Sharabasy, S., and M.M. El-Dawayati. 2017. Bioreactor steroid production and analysis of date palm embryogenic callus. In Date palm biotechnology protocols, vol. I, ed. J.M. Al-Khayri, S.M. Jain, and D. Johnson, 309–318., Tissue culture and applications New York: Humana Press.
El-Sharabasy, S.F. 2000. Studies on the production of secondary metabolites from date palm by using tissue culture technique. Ph.D. Thesis, Al-Azhar University, Cairo, p. 200.
Gantait, S., M.M. El-Dawayati, J. Panigrahi, C. Labrooy, and S.K. Verma. 2018. The retrospect and prospect of the applications of biotechnology in Phoenix dactylifera L. Applied Microbiology and Biotechnology 102: 8229–8259.
Irshad, M., B. Debnath, S. Mitra, Y. Arafat, M. Li, Y. Sun, and D. Qiu. 2018. Accumulation of anthocyanin in callus cultures of red-pod okra [Abelmoschus esculentus (L.) Hongjiao] in response to light and nitrogen levels. Plant Cell, Tissue and Organ Culture 134: 29–39.
Jovanović-Šanta, S.S., E.T. Petri, O.R. Klisurić, M. Szécsi, R. Kovačević, and J.A. Petrović. 2015. Antihormonal potential of selected D-homo and D-seco estratriene derivatives. Steroids 97: 45–53.
Kintzios, S., J. Drossopoulos, G. Sarlis, and J. Konstas. 2001. The effect of light intensity and relative exposure under light on the expression of direct or indirect somatic embryogenesis from common mallow (Malva sylvestris L.). International Conference on Medicinal and Aromatic Plants (Part II) 597: 315–319.
Kintzios, S., A. Nikolaou, and M. Skoula. 1999. Somatic embryogenesis and in vitro rosmarinic acid accumulation in Salvia officinalis and S. fruticosa leaf callus cultures. Plant Cell Reports 18: 462–466.
Kong, D.X., Y.Q. Li, M.L. Wang, M. Bai, R. Zou, H. Tang, and H. Wu. 2016. Effects of light intensity on leaf photosynthetic characteristics, chloroplast structure, and alkaloid content of Mahonia bodinieri (Gagnep.) Laferr. Acta Physiologiae Plantarum 38: 120.
Liu, Y., L. Song, W. Yu, Y. Hu, X. Ma, J. Wu, and Y. Ying. 2015. Light quality modifies camptothecin production and gene expression of biosynthesis in Camptotheca acuminata Decne seedlings. Industrial Crops and Products 66: 137–143.
Liu, Z., J.L. Qi, L. Chen, M.S. Zhang, X.Q. Wang, Y.J. Pang, and Y.H. Yang. 2006. Effect of light on gene expression and shikonin formation in cultured Onosma paniculatum cells. Plant Cell, Tissue and Organ Culture 84: 38–44.
Malik, S., S. Bhushan, M. Sharma, and P.S. Ahuja. 2016. Biotechnological approaches to the production of shikonins: a critical review with recent updates. Critical Reviews in Biotechnology 36: 327–340.
Mazri, M.A., R. Meziani, J. El Fadile, and A.E. Ezzinbi. 2016. Optimization of medium composition for in vitro shoot proliferation and growth of date palm cv. Mejhoul. 3 Biotech 6: 111.
Murashige, T., and F. Skoog. 1962. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiologiae Plantarum 15: 473–495.
Murthy, H.N., E.J. Lee, and K.Y. Paek. 2014. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell, Tissue and Organ Culture 118: 1–6.
Naik, P.M., and J.M. Al-Khayri. 2017. Extraction and estimation of secondary metabolites from date palm cell suspension cultures. In Date Palm Biotechnology Protocols, vol. I, ed. J.M. Al-Khayri, S.M. Jain, and D. Johnson, 319–332., Methods in Molecular Biology, Vol 1637 New York: Humana Press.
Ouzounis, T., B.R. Parjikolaei, X. Fretté, E. Rosenqvist, and C.O. Ottosen. 2015. Predawn and high intensity application of supplemental blue light decreases the quantum yield of PSII and enhances the amount of phenolic acids, flavonoids, and pigments in (Lactuca sativa). Frontiers in Plant Science 6: 19.
Patil, K.S., and S.R. Bhalsing. 2016. Effect of sugars on production of β-sitosterol from in vitro callus culture of (Boerhaavia diffusa L.). Acta Biologica Szegediensis 60: 99–104.
Patra, B., C. Schluttenhofer, Y. Wu, S. Pattanaik, and L. Yuan. 2013. Transcriptional regulation of secondary metabolite biosynthesis in plants. Biochimica et Biophysica Acta 1829: 1236–1247.
Pedroso, R.C.N., N.A.A. Branquinho, A.C.B.A.M. Hara, A.C. Costa, F.G. Silva, L.P. Pimenta, M.L.A. Silva, W.R. Cunha, P.M. Pauletti, and A.H. Januario. 2017. Impact of light quality on flavonoid production and growth of (Hyptis marrubioides) seedlings cultivated in vitro. Revista Brasileira de Farmacognosia 27: 466–470.
Saputro, A.D., D. Van de Walle, and K. Dewettinck. 2019. Palm sap sugar: a review. Sugar Tech 21: 862–867.
Sawwan, J.S., A.M. Al-Abdallat, T.S. Al-Qudah, M.M. Kushad, and M. Zucoloto. 2016. Hawthorn (Crataegus aronia L.) callus growth dynamics and polyphenol production under different light intensities. Journal of Food, Agriculture and Environment 14: 40–45.
Sultan, A., and A.R. Raza. 2015. Steroids: a diverse class of secondary metabolites. Medicinal Chemistry 5: 310–317.
Verma, S.K., S. Gantait, B.R. Jeong, and S.J. Hwang. 2018. Enhanced growth and cardenolides production in Digitalis purpurea under the influence of different LED exposures in the plant factory. Scientific Reports 8: 18009.
Yang, L., K.S. Wen, X. Ruan, Y.X. Zhao, F. Wei, and Q. Wang. 2018. Response of plant secondary metabolites to environmental factors. Molecules 23: 762.
Zayed, Z.E. 2017. Enhanced indirect somatic embryogenesis from shoot-tip explants of date palm by gradual reductions of 2,4-D concentration. In Date Palm Biotechnology Protocols, vol. I, ed. J. Al-Khayri, S. Jain, and D. Johnson, 77–88., Methods in molecular biology, vol 1637 New York: Humana Press.
Acknowledgements
The authors are thankful to the Central Laboratory of Date Palm Researches and Development, Agriculture Research Center, Giza, Egypt for providing research facilities. We further are grateful to the anonymous reviewers and the editor for their valuable comments and suggestions on the manuscript.
Funding
Self-funded.
Author information
Authors and Affiliations
Contributions
MME and SE conceived the research idea and designed the experiments; MME executed the experimental works; MME and SG contributed to data analysis, interpretation, and initial drafting of manuscript; MME, SE, and SG reviewed and edited the manuscript critically for important intellectual content. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Dawayati, M.M., El-Sharabasy, S. & Gantait, S. Light Intensity-Induced Morphogenetic Response and Enhanced β-Sitosterol Accumulation in Date Palm (Phoenix dactylifera L. cv. Hayani) Callus Culture. Sugar Tech 22, 1122–1129 (2020). https://doi.org/10.1007/s12355-020-00844-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-020-00844-9