Abstract
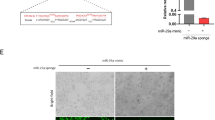
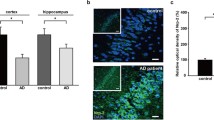
MicroRNAs have emerged as regulators of brain development and function. Reduction of miR-101 expression has been reported in rodent hippocampus during ageing, in the brain of Alzheimer’s disease (AD) patients and in AD animal models. In this study, we investigated the behavioral and molecular consequences of inhibition of endogenous miR-101 in 4–5-month-old C57BL/6J mice, infused with lentiviral particles expressing a miR-101 sponge (pLSyn-miR-101 sponge) in the CA1 field of the hippocampus. The sponge-infected mouse model showed cognitive impairment. The pLSyn-miR-101 sponge-infected mice were unable to discriminate either a novel object location or a novel object as assessed by object place recognition (OPR) and novel object recognition (NOR) tasks, respectively. Moreover, the sponge-infected mice evaluated for contextual memory in inhibitory avoidance task showed shorter retention latency compared to control pLSyn mice. These cognitive impairment features were associated with increased hippocampal expression of relevant miR-101 target genes, amyloid precursor protein (APP), RanBP9 and Rab5 and overproduction of amyloid beta (Aβ) 42 levels, the more toxic species of Aβ peptide. Notably, phosphorylation-dependent AMP-activated protein kinase (AMPK) hyperactivation is associated with AD pathology and age-dependent memory decline, and we found AMPK hyperphosphorylation in the hippocampus of pLSyn-miR-101 sponge mice. This study demonstrates that mimicking age-associated loss of miR-101 in hippocampal neurons induces cognitive decline and modulation of AD-related genes in mice.





Similar content being viewed by others
References
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer's disease at 25 years. J EMBO Mol Med 8(6):595–608. https://doi.org/10.15252/emmm.201606210
Bishop N, Lu T, Yankner B (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535. https://doi.org/10.1038/nature08983
Fan X, Wheatley EG, Villeda SA (2017) Mechanisms of hippocampal aging and the potential for rejuvenation. Annu Rev Neurosci 40:251–272. https://doi.org/10.1146/annurev-neuro-072116-031357
Wang M, Qin L, Tang B (2019) MicroRNAs in Alzheimer's disease. Front Genet 10:153. https://doi.org/10.3389/fgene.2019.00153
Bartel DP (2018) Metazoan MicroRNAs. Cell 17:20–51. https://doi.org/10.1016/j.cell.2018.03.006
Hu Z, Li Z (2017) miRNAs in synapse development and synaptic plasticity. Curr Opin Neurobiol 45:24–31. https://doi.org/10.1016/j.conb.2017.02.014
Hébert SS, Horré K, Nicolaï L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A et al (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A 105:6415–6420. https://doi.org/10.1073/pnas.0710263105
Nunez-Iglesias J, Liu CC, Morgan TE, Finch CE, Zhou XJ (2010) Joint genome-wide profiling of miRNA and mRNA expression in Alzheimer's disease cortex reveals altered miRNA regulation. PLoS One 5(2):e8898. https://doi.org/10.1371/journal.pone.0008898
Burgos K, Malenica I, Metpally R, Courtright A, Rakela B, Beach T, Shill H, Adler C et al (2014) Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer's and Parkinson's diseases correlate with disease status and features of pathology. PLoS One 9(5):e94839. https://doi.org/10.1371/journal.pone.0094839
Lippi G, Fernandes CC, Ewell LA, John D, Romoli B, Curia G, Taylor SR, Frady EP et al (2016) MicroRNA-101 regulates multiple developmental programs to constrain excitation in adult neural networks. Neuron 92(6):1337–1351. https://doi.org/10.1016/j.neuron.2016.11.017
Barak B, Shvarts-Serebro I, Modai S, Gilam A, Okun E, Michaelson DM, Mattson MP, Shomron N et al (2013) Opposing actions of environmental enrichment and Alzheimer's disease on the expression of hippocampal microRNAs in mouse models. Transl Psychiatry 3:e304. https://doi.org/10.1038/tp.2013.77
Che H, Sun LH, Guo F, Niu HF, Su XL, Bao YN, Fu ZD, Liu HL et al (2014) Expression of amyloid-associated miRNAs in both the forebrain cortex and hippocampus of middle-aged rat. Cell Physiol Biochem 33:11–22. https://doi.org/10.1159/000356646
Mohammed CP, Rhee H, Phee BK, Kim K, Kim HJ, Lee H, Park JH, Jung JH et al (2016) miR-204 downregulates EphB2 in aging mouse hippocampal neurons. Aging Cell 15:380–388. https://doi.org/10.1111/acel.12444
Wang X, Liu P, Zhu H, Xu Y, Ma C, Dai X, Huang L, Liu Y et al (2009) miR-34a, a microRNA up-regulated in a double transgenic mouse model of Alzheimer's disease, inhibits bcl2 translation. Brain Res Bull 80:268–273. https://doi.org/10.1016/j.brainresbull.2009.08.006
Vilardo E, Barbato C, Ciotti MT, Cogoni C, Ruberti F (2010) MicroRNA-101 regulates amyloid precursor protein expression in hippocampal neurons. J Biol Chem 285:18344–18351. https://doi.org/10.1074/jbc.M110.112664
Barbato C, Pezzola S, Caggiano C, Antonelli M, Frisone P, Ciotti MT, Ruberti F (2014) A lentiviral sponge for miR-101 regulates RanBP9 expression and amyloid precursor protein metabolism in hippocampal neurons. Front Cell Neurosci 8:37. https://doi.org/10.3389/fncel.2014.00037
Long JM, Lahiri DK (2011) MicroRNA-101 downregulates Alzheimer's amyloid-β precursor protein levels in human cell cultures and is differentially expressed. Biochem Biophys Res Commun 404:889–895. https://doi.org/10.1016/j.bbrc.2010.12.053
Liu D, Tang H, Li XY, Deng MF, Wei N, Wang X, Zhou YF, Wang DQ et al (2017) Targeting the HDAC2/HNF-4A/miR-101b/AMPK pathway rescues Tauopathy and dendritic abnormalities in Alzheimer's disease. Mol Ther 25:752–764. https://doi.org/10.1016/j.ymthe.2017.01.018
Coccurello R, Adriani W, Oliverio A, Mele A (2000) Effect of intra-accumbens dopamine receptor agents on reactivity to spatial and non-spatial changes in mice. Psychopharmacology 152:189–199
Roullet P, Sargolini F, Oliverio A, Mele A (2001) NMDA and AMPA antagonist infusions into the ventral striatum impair different steps of spatial information processing in a nonassociative task in mice. J Neurosci 21:2143–2149
Coccurello R, Oliverio A, Mele A (2012) Dopamine-glutamate interplay in the ventral striatum modulates spatial learning in a receptor subtype-dependent manner. Neuropsychopharmacology 37:1122–1133. https://doi.org/10.1038/npp.2011.296
Teich AF, Patel M, Arancio O (2013) A reliable way to detect endogenous murine β-amyloid. PLoS One 8:e55647. https://doi.org/10.1371/journal.pone.0055647
Ebert MS, Neilson JR, Sharp PA (2007) MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Methods 4:721–726
D'Hooge R, Nagels G, Westland CE, Mucke L, De Deyn PP (1996) Spatial learning deficit in mice expressing human 751-amino acid beta-amyloid precursor protein. Neuroreport. 7:2807–2811
Simón AM, Schiapparelli L, Salazar-Colocho P, Cuadrado-Tejedor M, Escribano L, López de Maturana R, Del Río J, Pérez-Mediavilla A et al (2009) Overexpression of wild-type human APP in mice causes cognitive deficits and pathological features unrelated to Abeta levels. Neurobiol Dis 33:369–378. https://doi.org/10.1016/j.nbd.2008.11.005
Woo JA, Jung AR, Lakshmana MK, Bedrossian A, Lim Y, Bu JH, Park SA, Koo EH et al (2012) Pivotal role of the RanBP9-cofilin pathway in Aβ-induced apoptosis and neurodegeneration. Cell Death Differ 19:1413–1423. https://doi.org/10.1038/cdd.2012.14
Palavicini JP, Wang H, Minond D, Bianchi E, Xu S, Lakshmana MK (2014) RanBP9 overexpression down-regulates phospho-cofilin, causes early synaptic deficits and impaired learning, and accelerates accumulation of amyloid plaques in the mouse brain. J Alzheimers Dis 39:727–740. https://doi.org/10.3233/JAD-131550
Frankel LB, Wen J, Lees M, Høyer-Hansen M, Farkas T, Krogh A, Jäättelä M, Lund AH (2011) microRNA-101 is a potent inhibitor of autophagy. EMBO J 30:4628–4641. https://doi.org/10.1038/emboj.2011.331
Ginsberg SD, Mufson EJ, Counts SE, Wuu J, Alldred MJ, Nixon RA, Che S (2010) Regional selectivity of rab5 and rab7 protein upregulation in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis 22:631–639. https://doi.org/10.3233/JAD-2010-101080
Näslund J, Haroutunian V, Mohs R, Davis KL, Davies P, Greengard P, Buxbaum JD (2000) Correlation between elevated levels of amyloid beta-peptide in the brain and cognitive decline. JAMA 283:1571–1577
Billings LM, Oddo S, Green KN, McGaugh JL, LaFerla FM (2005) Intraneuronal Abeta causes the onset of early Alzheimer's disease-related cognitive deficits in transgenic mice. Neuron 45:675–688
Lesné SE, Sherman MA, Grant M, Kuskowski M, Schneider JA, Bennett DA, Ashe KH (2013) Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain 136:1383–1398. https://doi.org/10.1093/brain/awt062
Garcia-Osta A, Alberini CM (2009) Amyloid beta mediates memory formation. Learn Mem 16(4):267–272. https://doi.org/10.1101/lm.1310209
Puzzo D, Privitera L, Fa M, Staniszewski A, Hashimoto G, Aziz F, Sakurai M, Ribe EM et al (2011) Endogenous amyloid-β is necessary for hippocampal synaptic plasticity and memory. Ann Neurol 69:819–830. https://doi.org/10.1002/ana.22313
Gulisano W, Melone M, Ripoli C, Tropea MR, Li Puma DD, Giunta S, Cocco S, Marcotulli D et al (2019) Neuromodulatory action of picomolar extracellular Aβ42 oligomers on presynaptic and postsynaptic mechanisms underlying synaptic function and memory. J Neurosci 39:5986–6000. https://doi.org/10.1523/JNEUROSCI.0163-19.2019
Vingtdeux V, Davies P, Dickson DW, Marambaud P (2011) AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer's diseaseand other tauopathies. Acta Neuropathol 121:337–349. https://doi.org/10.1007/s00401-010-0759-x
Mairet-Coello G, Courchet J, Pieraut S, Courchet V, Maximov A, Polleux F (2013) The CAMKK2-AMPK kinase pathway mediates the synaptotoxic effects of Aβ oligomers through tau phosphorylation. Neuron 78(1):94–108. https://doi.org/10.1016/j.neuron.2013.02.003
Ma T, Chen Y, Vingtdeux V, Zhao H, Viollet B, Marambaud P, Klann E (2014) Inhibition of AMP-activated protein kinase signaling alleviates impairments in hippocampal synaptic plasticity induced by amyloid β. J Neurosci 34(36):12230–12238. https://doi.org/10.1523/JNEUROSCI.1694-14.2014
Yang W, Zhou X, Ma T (2019) Memory decline and behavioral inflexibility in aged mice are correlated with dysregulation of protein synthesis capacity. Front Aging Neurosci 11:246. https://doi.org/10.3389/fnagi.2019.00246 eCollection 2019
Han Y, Luo Y, Sun J, Ding Z, Liu J, Yan W, Jian M, Xue Y et al (2016) AMPK signaling in the dorsal Hippocampus negatively regulates contextual fear memory formation. Neuropsychopharmacology 41:1849–1864. https://doi.org/10.1038/npp.2015.355
Wimmer ME, Hernandez PJ, Blackwell J, Abel T (2012) Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiol Aging 33:2220–2224. https://doi.org/10.1016/j.neurobiolaging.2011.07.007
Kim DH, Jang YS, Jeon WK, Han JS (2019) Assessment of cognitive phenotyping in inbred, genetically modified mice, and transgenic mouse models of Alzheimer's disease. Exp Neurobiol 28:146–157. https://doi.org/10.5607/en.2019.28.2.146
Zhang R, Xue G, Wang S, Zhang L, Shi C, Xie X (2012) Novel object recognition as a facile behavior test for evaluating drug effects in AβPP/PS1 Alzheimer's disease mouse model. J Alzheimers Dis 31:801–812. https://doi.org/10.3233/JAD-2012-120151
Creighton SD, Mendell AL, Palmer D, Kalisch BE, MacLusky NJ, Prado VF, Prado MAM, Winters BD (2019) Dissociable cognitive impairments in two strains of transgenic Alzheimer's disease mice revealed by a battery of object-based tests. Sci Rep 9:57. https://doi.org/10.1038/s41598-018-37312-0
Ameen-Ali KE, Simpson JE, Wharton SB, Heath PR, Sharp PS, Brezzo G, Berwick J (2019) The time course of recognition memory impairment and glial pathology in the hAPP-J20 mouse model of Alzheimer's disease. J Alzheimers Dis 68:609–624. https://doi.org/10.3233/JAD-181238
Cohen JL, Jackson NL, Ballestas ME, Webb WM, Lubin FD, Clinton SM (2017) Amygdalar expression of the microRNA miR-101a and its target Ezh2 contribute to rodent anxiety-like behaviour. Eur J Neurosci 46:2241–2252. https://doi.org/10.1111/ejn.13624
Zhao Y, Wang S, Chu Z, Dang Y, Zhu J, Su X (2017) MicroRNA-101 in the ventrolateral orbital cortex (VLO) modulates depressive-like behaviors in rats and targets dual-specificity phosphatase 1 (DUSP1). Brain Res 1669:55–62. https://doi.org/10.1016/j.brainres.2017.05.020
Woo JA, Boggess T, Uhlar C, Wang X, Khan H, Cappos G, Joly-Amado A, De Narvaez E et al (2015) RanBP9 at the intersection between cofilin and Aβ pathologies: rescue of neurodegenerative changes by RanBP9 reduction. Cell Death Dis 6:1676. https://doi.org/10.1038/cddis.2015.37
Knobloch M, Konietzko U, Krebs DC, Nitsch RM (2007) Intracellular Abeta and cognitive deficits precede beta-amyloid deposition in transgenic arcAbeta mice. Neurobiol Aging 28:1297–1306
Ochiishi T, Kaku M, Kiyosue K, Doi M, Urabe T, Hattori N, Shimura H, Ebihara T (2019) New Alzheimer's disease model mouse specialized for analyzing the function and toxicity of intraneuronal amyloid β oligomers. Sci Rep 9:17368. https://doi.org/10.1038/s41598-019-53415-8
Funding
This work was supported by the Consiglio Nazionale delle Ricerche, Aging Programme DSB.AD009.001.004 IBCN Workpackage 1.9 “Study of molecular and cellular mechanisms involved in the pathogenesis and progression of Alzheimer’s Disease” to Francesca Ruberti & Christian Barbato. The Italian Ministry of Health (Ricerca Corrente) also supported the work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 3.06 mb)
Rights and permissions
About this article
Cite this article
Barbato, C., Giacovazzo, G., Albiero, F. et al. Cognitive Decline and Modulation of Alzheimer’s Disease-Related Genes After Inhibition of MicroRNA-101 in Mouse Hippocampal Neurons. Mol Neurobiol 57, 3183–3194 (2020). https://doi.org/10.1007/s12035-020-01957-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-01957-8