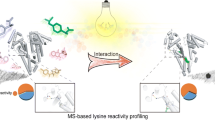
Abstract
Modification of endogenous proteins by drugs and drug metabolites are thought to be a cause of idiosyncratic adverse drug reactions (IADRs). Trimethoprim (TMP) is a commonly prescribed antibiotic that has been implicated in IADRs; however, there is no known mechanism by which this drug or its metabolites modify proteins. This study describes the results of screening trimethoprim and its primary metabolites for the ability to covalently modify human serum albumin (HSA). The first step of the screen was in vitro reactions of the compounds with HSA followed by western blotting with antisera specific to drug-modified proteins. Compounds with positive signal in the western blot were then screened using an untargeted peptide profiling method to discover modified peptides. This strategy identified two sites in HSA that are modified by incubation with a TMP metabolite, α-hydroxy trimethoprim (Cα-OH-TMP).






Similar content being viewed by others
Abbreviations
- (TMP):
-
trimethoprim
- (IADR):
-
idiosyncratic adverse drug reactions
- (HLM):
-
human liver microsomal
- (NAC):
-
N-acetylcysteine
- (1-NO-TMP):
-
1-N-oxide trimethoprim
- (3-NO-TMP):
-
3-N-oxide trimethoprim
- (3-DM-TMP):
-
3ʹ-desmethyl-trimethoprim
- (4-DM-TMP):
-
4ʹ-desmethyl-trimethoprim
- (Cα-OH-TMP):
-
α-hydroxy trimethoprim
- CBZE:
-
carbamazepine 10,11-epoxide
- HSA:
-
human serum albumin
- (KLH):
-
keyhole limpet hemocyanin
- (LC-MS):
-
liquid chromatography mass spectrometry
- (EIC):
-
extracted ion chromatogram
References
Aldini G, Gamberoni L, Orioli M, Beretta G, Regazzoni L, Maffei Facino R, Carini M (2006) Mass spectrometric characterization of covalent modification of human serum albumin by 4-hydroxy-trans-2-nonenal. J Mass Spectrom 41:1149–1161
Chalkley RJ, Clauser KR (2012) Modification site localization scoring: strategies and performance. Mol Cell Proteom 11:3–14
Cho T, Uetrecht J (2017) How reactive metabolites induce an immune response that sometimes leads to an idiosyncratic drug reaction. Chem Res Toxicol 30:295–314
Damsten MC, de Vlieger JS, Niessen WM, Irth H, Vermeulen NP, Commandeur JN (2008) Trimethoprim: novel reactive intermediates and bioactivation pathways by cytochrome p450s. Chem Res Toxicol 21:2181–2187
Das G, Bailey MJ, Wickham JE (1988) Toxic epidermal necrolysis and trimethoprim. Br Med J 296:1604–1605
Evans DC, Watt AP, Nicoll-Griffith DA, Baillie TA (2004) Drug-protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem Res Toxicol 17(1):3–16
Goldman JL, Koen YM, Rogers SA, Li K, Leeder JS, Hanzlik RP (2016) Bioactivation of trimethoprim to protein-reactive metabolites in human liver microsomes. Drug Metab Dispos 44:1603–1607
Goldman JL, Leeder JS, Van Haandel L, Pearce RE (2015) In vitro hepatic oxidative biotransformation of trimethoprim. Drug Metab Dispos 43:1372–1380
Goldman JL, van Haandel L (2018) Trimethoprim: the overlooked component of trimethoprim-sulfamethoxazole idiosyncratic adverse drug reactions. Pharmacoepidemiol. drug Saf 27:949–951
Hawkins T, Carter JM, Romeril KR, Jackson SR, Green GJ (1993) Severe trimethoprim induced neutropenia and thrombocytopenia. NZ Med J 106:251–252
Higgins T, Niklasson PM (1990) Hypersensitivity pneumonitis induced by trimethoprim. BMJ 300:1344
Hinneburg H, Stavenhagen K, Schweiger-Hufnagel U, Pengelley S, Jabs W, Seeberger PH, Silva DV, Wuhrer M, Kolarich D (2016) The art of destruction: optimizing collision energies in quadrupole-time of flight (Q-TOF) instruments for glycopeptide-based glycoproteomics. J Am Soc Mass Spectrom 27:507–519
Lai WG, Zahid N, Uetrecht JP (1999) Metabolism of trimethoprim to a reactive iminoquinone methide by activated human neutrophils and hepatic microsomes. J Pharm Exp Ther 291:292–299
Meng X, Al-Attar Z, Yaseen FS, Jenkins R, Earnshaw C, Whitaker P, Peckham D, French NS, Naisbitt DJ, Park BK (2017) Definition of the nature and hapten threshold of the beta-lactam antigen required for T cell activation in vitro and in patients. J Immunol 198:4217–4227
Meng X, Maggs JL, Usui T, Whitaker P, French NS, Naisbitt DJ, Park BK (2015) Auto-oxidation of isoniazid leads to isonicotinic-lysine adducts on human serum albumin. Chem Res Toxicol 28:51–58
Nakayama S, Atsumi R, Takakusa H, Kobayashi Y, Kurihara A, Nagai Y, Nakai D, Okazaki O (2009) A zone classification system for risk assessment of idiosyncratic drug toxicity using daily dose and covalent binding. Drug Metab Dispos 37(9):1970–1977
Nolte WM, Tagore DM, Lane WS, Saghatelian A (2009) Peptidomics of prolyl endopeptidase in the central nervous system. Biochemistry 48:11971–11981
Sabbioni G, Turesky RJ (2017) Biomonitoring human albumin adducts: the past, the present, and the future. Chem Res Toxicol 30:332–366
Sanderson JP, Naisbitt DJ, Farrell J, Ashby CA, Tucker MJ, Rieder MJ, Pirmohamed M, Clarke SE, Park BK (2007) Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J Immunol 178:5533–5542
Sigel CW, Grace ME, Nichol CA (1973) Metabolism of trimethoprim in man and measurement of a new metabolite: a new fluorescence assay. J Infect Dis 128(Suppl):580–583
Stewart AJ, Blindauer CA, Berezenko S, Sleep D, Tooth D, Sadler PJ (2005) Role of Tyr84 in controlling the reactivity of Cys34 of human albumin. FEBS J 272:353–362
Tautenhahn R, Patti GJ, Rinehart D, Siuzdak G (2012) XCMS Online: a web-based platform to process untargeted metabolomic data. Anal Chem 84:5035–5039
Tran NH, Qiao R, Xin L, Chen X, Liu C, Zhang X, Shan B, Ghodsi A, Li M (2019) Deep learning enables de novo peptide sequencing from data-independent-acquisition mass spectrometry. Nat methods 16:63–66
Uetrecht J, Naisbitt DJ (2013) Idiosyncratic adverse drug reactions: current concepts. Pharmacol Rev 65:779–808
van Haandel L, Goldman JL, Pearce RE, Leeder JS (2014) Urinary biomarkers of trimethoprim bioactivation in vivo following therapeutic dosing in children. Chem Res Toxicol 27:211–218
Wijkstrom A, Westerlund D (1983) Plasma protein binding of sulphadiazine, sulphamethoxazole and trimethoprim determined by ultrafiltration. J Pharm Biomed Anal 1:293–299
Yip VLM, Meng X, Maggs JL, Jenkins RE, Marlot PT, Marson AG, Park BK, Pirmohamed M (2017) Mass spectrometric characterization of circulating covalent protein adducts derived from epoxide metabolites of carbamazepine in patients. Chem Res Toxicol 30:1419–1435
Acknowledgements
We would like to thank Robert Hanzlik and Yakov Koen at the University of Kansas, Department of Medicinal Chemistry, for their assistance in TMP antigen generation and antisera dilution and inhibition work. This work was supported by the National Institutes of Health R01GM129783 (J.L.G).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nolte, W.M., Tessman, R.T. & Goldman, J.L. Screening trimethoprim primary metabolites for covalent binding to albumin. Med Chem Res 29, 1238–1246 (2020). https://doi.org/10.1007/s00044-020-02570-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-020-02570-z