Abstract
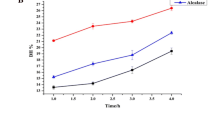
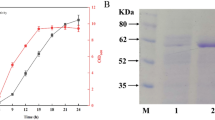
Microbial proteases are relevant biocatalysts with diverse applications. Production of protein hydrolysates is recently focused, since they might display biological activities. Therefore, the extracellular protease from Bacillus sp. CL18 was partially purified through ammonium sulfate precipitation (25–50% saturation) and gel filtration chromatography, with a 60.7-fold purification (40,593 U/mg protein) and 21.3% recovery. The partially purified protease (PPP) was characterized as a serine protease, with optimal activity at 51–59 °C and pH 7.4–8.8 and low thermal stability. Thermal inactivation followed first-order kinetics. PPP depended on Ca2+ for higher thermal stability, depicted by increases in half-lives (t1/2), activation energy (Ea), and free energy (ΔG#) for kinetic inactivation. PPP preferentially hydrolyzed casein > soy protein isolate (SPI) >>> keratinous materials. SPI hydrolysis by PPP was further investigated, and the obtained hydrolysates exhibited increased in vitro bioactivities. Hydrolysates displayed antioxidant capacities through the scavenging of synthetic organic radicals and Fe3+-reducing ability. In addition, hydrolysates inhibited the activities of dipeptidyl peptidase IV (DPP IV) and angiotensin-converting enzyme (ACE), suggesting antidiabetic and antihypertensive potentials, respectively. From its biochemical properties, PPP might be used to produce protein hydrolysates with multifunctional bioactivities. Both PPP and SPI hydrolysates can find applications in food biotechnology.




Similar content being viewed by others
Abbreviations
- A/A0 :
-
Residual activity during thermal inactivation of partially purified protease
- Abs:
-
Absorbance
- ΔG# :
-
Free energy change for the thermal inactivation of partially purified protease
- ΔH# :
-
Enthalpy change for the thermal inactivation of partially purified protease
- ΔS# :
-
Entropy change for the thermal inactivation of the partially purified protease
- DMSO:
-
Dimethyl sulfoxide
- E a :
-
Activation energy for thermal inactivation of partially purified protease
- EDTA:
-
Ethylenediaminetetraacetic acid
- h :
-
Planck constant (6.6262 × 10−34 J/s)
- k :
-
Inactivation rate of the partially purified protease at a given temperature
- K b :
-
Boltzmann constant (1.3806 × 10−23 J/K)
- SPI:
-
Soy protein isolate
- HSPI:
-
SPI hydrolysate
- HSPI2:
-
HSPI obtained through hydrolysis with 2% (v/v) of partially purified protease
- HSPI4:
-
HSPI obtained through hydrolysis with 4% (v/v) of partially purified protease
- P :
-
Proteolytic activity predicted by a second-order mathematical model
- PMSF:
-
Phenylmethylsulfonyl fluoride
- PPP:
-
Partially purified protease
- R :
-
Universal gas constant [8.314 J/(mol K)]
- SDS:
-
Sodium dodecyl sulfate
- SDS-PAGE:
-
SDS-polyacrylamide gel electrophoresis
- t :
-
Time
- T :
-
temperature
- t 1/2 :
-
Half-life of PPP at a given temperature
- TCA:
-
Trichloroacetic acid
- U:
-
Unit of protease activity
References
Gurumallesh, P., Alagu, K., Ramakrishnan, B., & Muthusamy, S. (2019). A systematic reconsideration on proteases. International Journal of Biological Macromolecules, 128, 254–267. https://doi.org/10.1016/j.ijbiomac.2019.01.081.
Razzaq, A., Shamsi, S., Ali, A., Ali, Q., Sajjad, M., Malik, A., & Ashraf, M. (2019). Microbial proteases applications. Frontiers in Bioengineering and Biotechnology, 7, 110. https://doi.org/10.3389/fbioe.2019.00110.
Kamal, S., Rehman, S., & Iqbal, H. M. N. (2017). Biotechnological valorization of proteases: from hyperproduction to industrial exploitation – a review. Environmental Progress & Sustainable Energy, 36(2), 511–522. https://doi.org/10.1002/ep.12447.
Contesini, F. J., Melo, R. R., & Sato, H. H. (2018). An overview of Bacillus proteases: from production to application. Critical Reviews in Biotechnology, 38(3), 321–334. https://doi.org/10.1080/07388551.2017.1354354.
Silva, R. R. (2017). Bacterial and fungal proteolytic enzymes: production, catalysis and potential applications. Applied Biochemistry and Biotechnology, 183(1), 1–19. https://doi.org/10.1007/s12010-017-2427-2.
Aluko, R. E. (2015). Antihypertensive peptides from food proteins. Annual Review of Food Science and Technology, 6(1), 235–262. https://doi.org/10.1146/annurev-food-022814-015520.
Lacroix, I. M. E., & Li-Chan, E. C. Y. (2016). Food-derived dipeptidyl-peptidase IV inhibitors as a potential approach for glycemic regulation – current knowledge and future research considerations. Trends in Food Science & Technology, 54, 1–16. https://doi.org/10.1016/j.tifs.2016.05.008.
Nwachukwu, I. D., & Aluko, R. E. (2019). Structural and functional properties of food protein-derived antioxidant peptides. Journal of Food Biochemistry, 43(1), e12761. https://doi.org/10.1111/jfbc.12761.
Singh, B. P., Vij, S., & Hati, S. (2014). Functional significance of bioactive peptides derived from soybean. Peptides, 54, 171–179. https://doi.org/10.1016/j.peptides.2014.01.022.
Aguilar, J. G. S., & Sato, H. H. (2018). Microbial proteases: production and application in obtaining protein hydrolysates. Food Research International, 103, 253–262. https://doi.org/10.1016/j.foodres.2017.10.044.
Sobucki, L., Ramos, R. F., & Daroit, D. J. (2017). Protease production by the keratinolytic Bacillus sp. CL18 through feather bioprocessing. Environmental Science and Pollution Research, 24(29), 23125–23132. https://doi.org/10.1007/s11356-017-9876-6.
Daroit, D. J., Sant’Anna, V., & Brandelli, A. (2011). Kinetic stability modelling of keratinolytic protease P45: influence of temperature and metal ions. Applied Biochemistry and Biotechnology, 165(7-8), 1740–1753. https://doi.org/10.1007/s12010-011-9391-z.
Corrêa, A. P. F., Daroit, D. J., Coelho, J., Meira, S. M. M., Lopes, F. C., Segalin, J., Risso, P. H., & Brandelli, A. (2011). Antioxidant, antihypertensive and antimicrobial properties of ovine milk caseinate hydrolyzed with a microbial protease. Journal of the Science of Food and Agriculture, 91, 2247–2254. https://doi.org/10.1002/jsfa.4446.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685. https://doi.org/10.1038/227680a0.
Corrêa, A. P. F., Daroit, D. J., & Brandelli, A. (2010). Characterization of a keratinase produced by Bacillus sp. P7 isolated from an Amazonian environment. International Biodeterioration and Biodegradation, 64(1), 1–6. https://doi.org/10.1016/j.ibiod.2009.06.015.
Daroit, D. J., Corrêa, A. P. F., Segalin, J., & Brandelli, A. (2010). Characterization of a keratinolytic protease produced by the feather-degrading Amazonian bacterium Bacillus sp. P45. Biocatalysis and Biotransformation, 28(5-6), 370–379. https://doi.org/10.3109/10242422.2010.532549.
Re, R., Pellegrini, N., Proteggente, A., Pannala, A., Yang, M., & Rice-Evans, C. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology and Medicine, 26(9-10), 1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3.
Brand-Williams, W., Cuvelier, M. E., & Berset, C. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28, 25–30. https://doi.org/10.1016/S0023-6438(95)80008-5.
Callegaro, K., Welter, N., & Daroit, D. J. (2018). Feathers as bioresource: microbial conversion into bioactive protein hydrolysates. Process Biochemistry, 75, 1–9. https://doi.org/10.1016/j.procbio.2018.09.002.
Cushman, D. W., & Cheung, H. S. (1971). Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochemical Pharmacology, 20, 1637–1648. https://doi.org/10.1016/0006-2952(71)90292-9.
Zhang, Y., Chen, R., Ma, H., & Chen, S. (2015). Isolation and identification of dipeptidyl peptidase IV-inhibitory peptides from trypsin/chymotrypsin-treated goat milk casein hydrolysates by 2D-TLC and LC-MS/MS. Journal of Agricultural and Food Chemistry, 63, 8819–8828. https://doi.org/10.1021/acs.jafc.5b03062.
Bose, A., Pathan, S., Pathak, K., & Keharia, H. (2014). Keratinolytic protease production by Bacillus amyloliquefaciens 6B using feather meal as substrate and application of feather hydrolysate as organic nitrogen input for agricultural soil. Waste and Biomass Valorization, 5(4), 595–605. https://doi.org/10.1007/s12649-013-9272-5.
Ramkumar, A., Sivakumar, N., Gujarathi, A. M., & Victor, R. (2018). Production of thermotolerant, detergent stable alkaline protease using the gut waste of Sardinella longiceps as a substrate: optimization and characterization. Scientific Reports, 8(1), 12442. https://doi.org/10.1038/s41598-018-30155-9.
Rathod, M. G., & Pathak, A. P. (2016). Optimized production, characterization and application of alkaline proteases from taxonomically assessed microbial isolates from Lonar soda lake, India. Biocatalysis and Agricultural Biotechnology, 7, 164–173. https://doi.org/10.1016/j.bcab.2016.06.002.
Fakhfakh-Zouari, N., Hmidet, N., Haddar, A., Kanoun, S., & Nasri, M. (2010). A novel serine metallokeratinase from a newly isolated Bacillus pumilus A1 grown on chicken feather meal: biochemical and molecular characterization. Applied Biochemistry and Biotechnology, 162, 329–344. https://doi.org/10.1007/s12010-009-8774-x.
Bhange, K., Chaturvedi, V., & Bhatt, R. (2016). Simultaneous production of detergent stable keratinolytic protease, amylase and biosurfactant by Bacillus subtilis PF1 using agro industrial waste. Biotechnology Reports, 10, 94–104. https://doi.org/10.1016/j.btre.2016.03.007.
Anbu, P. (2013). Characterization of solvent stable extracellular protease from Bacillus koreensis (BK-P21A). International Journal of Biological Macromolecules, 56, 162–168. https://doi.org/10.1016/j.ijbiomac.2013.02.014.
Abu-Khudir, R., Salem, M. M., Allam, N. G., & Ali, E. M. M. (2019). Production, partial purification, and biochemical characterization of a thermotolerant alkaline metallo-protease from Staphylococcus sciuri. Applied Biochemistry and Biotechnology, 189(1), 87–102. https://doi.org/10.1007/s12010-019-02983-6.
Ferrareze, P. A. G., Corrêa, A. P. F., & Brandelli, A. (2016). Purification and characterization of a keratinolytic protease produced by probiotic Bacillus subtilis. Biocatalysis and Agricultural Biotechnology, 7, 102–109. https://doi.org/10.1016/j.bcab.2016.05.009.
Gohel, S. D., & Singh, S. P. (2018). Thermodynamics of a Ca2+ dependent, highly thermostable and detergent compatible purified alkaline serine protease from Nocardiopsis xinjiangensis strain OM-6. International Journal of Biological Macromolecules, 113, 565–574. https://doi.org/10.1016/j.ijbiomac.2018.02.157.
Silveira, S. T., Casarin, F., Gemelli, S., & Brandelli, A. (2010). Thermodynamics and kinetics of heat inactivation of a novel keratinase from Chryseobacterium sp. strain kr6. Applied Biochemistry and Biotechnology, 162(2), 548–560. https://doi.org/10.1007/s12010-009-8835-1.
Silveira, S. T., Daroit, D. J., Sant’Anna, V., & Brandelli, A. (2013). Stability modeling of red pigments produced by Monascus purpureus in submerged cultivations with sugarcane bagasse. Food and Bioprocess Technology, 6(4), 1007–1014. https://doi.org/10.1007/s11947-011-0710-8.
Gouzi, H., Depagne, C., & Coradin, T. (2012). Kinetics and thermodynamics of the thermal inactivation of polyphenol oxidase in an aqueous extract from Agaricus bisporus. Journal of Agricultural and Food Chemistry, 60(1), 500–506. https://doi.org/10.1021/jf204104g.
Silva, O. S., Oliveira, R. L., Silva, J. C., Converti, A., & Porto, T. S. (2018). Thermodynamic investigation of an alkaline protease from Aspergillus tamarii URM4634: a comparative approach between crude extract and purified enzyme. International Journal of Biological Macromolecules, 109, 1039–1044. https://doi.org/10.1016/j.ijbiomac.2017.11.081.
Hadjidj, R., Badis, A., Mechri, S., Eddouaouda, K., Khelouia, L., Annane, R., El Hattab, M., & Jaouadi, B. (2018). Purification, biochemical, and molecular characterization of novel protease from Bacillus licheniformis strain K7A. International Journal of Biological Macromolecules, 114, 1033–1048. https://doi.org/10.1016/j.ijbiomac.2018.03.167.
Xu, J., Zhuang, Y., Wu, B., Su, L., & He, B. (2013). Calcium-ion-induced stabilization of the protease from Bacillus cereus WQ9-2 in aqueous hydrophilic solvents: effect of calcium ion binding on the hydration shell and intramolecular interactions. Journal of Biological Inorganic Chemistry, 18(2), 211–221. https://doi.org/10.1007/s00775-012-0966-0.
Castro, R. J. S., & Sato, H. H. (2014). Antioxidant activities and functional properties of soy protein isolate hydrolysates obtained using microbial proteases. International Journal of Food Science and Technology, 49(2), 317–328. https://doi.org/10.1111/ijfs.12285.
Oliveira, C. F., Corrêa, A. P. F., Coletto, D., Daroit, D. J., Cladera-Olivera, F., & Brandelli, A. (2015). Soy protein hydrolysis with microbial protease to improve antioxidant and functional properties. Journal of Food Science and Technology, 52(5), 2668–2678. https://doi.org/10.1007/s13197-014-1317-7.
Oliveira, C. F., Coletto, D., Corrêa, A. P. F., Daroit, D. J., Toniolo, R., Cladera-Olivera, F., & Brandelli, A. (2014). Antioxidant activity and inhibition of meat lipid oxidation by soy protein hydrolysates obtained with a microbial protease. International Food Research Journal, 21, 775–781.
Meza-Espinoza, L., Sáyago-Ayerdi, S. G., García-Magaña, M. L., Tovar-Pérez, E. G., Yahia, E. M., Vallejo-Cordoba, B., González-Córdova, A. F., Hernández-Mendoza, A., & Montalvo-González, E. (2018). Antioxidant capacity of egg, milk and soy protein hydrolysates and biopeptides produced by Bromelia pinguin and Bromelia karatas-derived proteases. Emirates Journal of Food and Agriculture, 30, 122–130. https://doi.org/10.9755/ejfa.2018.v30.i2.1604.
Conti, J. P., Vinderola, G., & Esteban, E. N. (2019). Characterization of a soy protein hydrolyzate for the development of a functional ingredient. Journal of Food Science and Technology, 56(2), 896–904. https://doi.org/10.1007/s13197-018-3551-x.
Guan, H., Diao, X., Jiang, F., Han, J., & Kong, B. (2018). The enzymatic hydrolysis of soy protein isolate by Corolase PP under high hydrostatic pressure and its effect on bioactivity and characteristics of hydrolysates. Food Chemistry, 245, 89–96. https://doi.org/10.1016/j.foodchem.2017.08.081.
Coscueta, E. R., Amorim, M. M., Voss, G. B., Nerli, B. B., Picó, G. A., & Pintado, M. E. (2016). Bioactive properties of peptides obtained from Argentinian defatted soy flour protein by Corolase PP hydrolysis. Food Chemistry, 198, 36–44. https://doi.org/10.1016/j.foodchem.2015.11.068.
Zhang, Q., Tong, X., Sui, X., Wang, Z., Qi, B., Li, Y., & Jiang, L. (2018). Antioxidant activity and protective effects of Alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Research International, 111, 256–264. https://doi.org/10.1016/j.foodres.2018.05.046.
Jara, A. M. R., Liggieri, C. S., & Bruno, M. A. (2018). Preparation of soy protein hydrolysates with antioxidant activity by using peptidases from latex of Maclura pomifera fruits. Food Chemistry, 264, 326–333. https://doi.org/10.1016/j.foodchem.2018.05.013.
Fan, J., Saito, M., Yanyan, Z., Szesze, T., Wang, L., Tatsumi, E., & Li, L. (2005). Gel-forming ability and radical-scavenging activity of soy protein hydrolysate treated with transglutaminase. Journal of Food Science, 70(1), C87–C92. https://doi.org/10.1111/j.1365-2621.2005.tb09027.x.
Zhang, L., Li, J., & Zhou, K. (2010). Chelating and radical scavenging activities of soy protein hydrolysates prepared from microbial proteases and their effect on meat lipid peroxidation. Bioresource Technology, 101(7), 2084–2089. https://doi.org/10.1016/j.biortech.2009.11.078.
Jiménez-Ruiz, E. I., Barca, A. M. C., Sotelo-Mundo, R. R., Arteaga-Mackinney, G. E., Valenzuela-Melendez, M., & Peña-Ramos, E. A. (2013). Partial characterization of ultrafiltrated soy protein hydrolysates with antioxidant and free radical scavenging activities. Journal of Food Science, 78(8), C1152–C1158. https://doi.org/10.1111/1750-3841.12200.
Liu, P., Huang, M., Song, S., Hayat, K., Zhang, X., Xia, S., & Jia, C. (2012). Sensory characteristics and antioxidant activities of Maillard reaction products from soy protein hydrolysates with different molecular weight distribution. Food and Bioprocess Technology, 5(5), 1775–1789. https://doi.org/10.1007/s11947-010-0440-3.
Arise, A. K., Alashi, A. M., Nwachukwu, I. F., Ijabadeniyi, O. A., Aluko, R. E., & Amonsou, E. O. (2016). Antioxidant activities of bambara groundnut (Vigna subterranea) protein hydrolysates and their membrane ultrafiltration fractions. Food and Function, 7(5), 2431–2437. https://doi.org/10.1039/c6fo00057f.
Chiang, W.-D., Tsou, M.-J., Tsai, Z.-Y., & Tsai, T.-C. (2006). Angiotensin I-converting enzyme inhibitor derived from soy protein hydrolysate and produced by using membrane reactor. Food Chemistry, 98(4), 725–732. https://doi.org/10.1016/j.foodchem.2005.06.038.
Lee, J.-S., Yoo, M. A., Koo, S. H., Baek, H.-H., & Lee, H. G. (2008). Antioxidant and ACE inhibitory activities of soybean hydrolysates: effect of enzyme and degree of hydrolysis. Food Science and Biotechnology, 17, 873–877.
Rudolph, S., Lunow, D., Kaiser, S., & Henle, T. (2017). Identification and quantification of ACE-inhibiting peptides in enzymatic hydrolysates of plant proteins. Food Chemistry, 224, 19–25. https://doi.org/10.1016/j.foodchem.2016.12.039.
Wang, R., Zhao, H., Pan, X., Orfila, C., Lu, W., & Ma, Y. (2019). Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Science & Nutrition, 7(5), 1848–1856. https://doi.org/10.1002/fsn3.1038.
Daliri, E. B.-M., Ofosu, F. K., Chelliah, R., Park, M. H., Kim, J.-H., & Oh, D.-H. (2019). Development of a soy protein hydrolysate with an antihypertensive effect. International Journal of Molecular Sciences, 20(6), 1496. https://doi.org/10.3390/ijms20061496.
Cha, M., & Park, J. R. (2005). Production and characterization of a soy protein-derived angiotensin I-converting enzyme inhibitory hydrolysate. Journal of Medicinal Food, 8(3), 305–310. https://doi.org/10.1089/jmf.2005.8.305.
Kuba, M., Tana, C., Tawata, S., & Yasuda, M. (2005). Production of angiotensin I converting enzyme inhibitory peptides from soybean protein with Monascus purpureus acid proteinase. Process Biochemistry, 40(6), 2191–2196. https://doi.org/10.1016/j.procbio.2004.08.010.
Hsieh, C.-H., Wang, T.-Y., Hung, C.-C., Jao, C.-L., Hsieh, Y.-L., Wu, S.-X., & Hsu, K.-C. (2016). In silico, in vitro and in vivo analyses of dipeptidyl peptidase IV inhibitory activity and the antidiabetic effect of sodium caseinate hydrolysate. Food and Function, 7(2), 1122–1128. https://doi.org/10.1039/c5fo01324k.
Nongonierma, A. B., & FitzGerald, R. J. (2015). Investigation of the potential of hemp, pea, rice and soy protein hydrolysates as a source of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Digestion: Research and Current Opinion, 6(1-3), 19–29. https://doi.org/10.1007/s13228-015-0039-2.
Funding
This work was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq, Brazil (Grant number 402631/2016-1). N. J. Clerici and D. J. Daroit also thank CNPq and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul – FAPERGS (Brazil) for the Scientific Initiation scholarship grants (IC-CNPq and PROBIC-FAPERGS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
( 588 kb)
Rights and permissions
About this article
Cite this article
Lermen, A.M., Clerici, N.J. & Daroit, D.J. Biochemical Properties of a Partially Purified Protease from Bacillus sp. CL18 and Its Use to Obtain Bioactive Soy Protein Hydrolysates. Appl Biochem Biotechnol 192, 643–664 (2020). https://doi.org/10.1007/s12010-020-03355-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-020-03355-1