Abstract
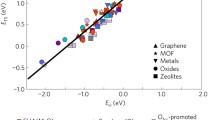
Recent developments in computational catalysis have allowed the routine reduction of the dimensionality of complex reaction networks to a few descriptors based on linear scaling relations. Despite this convenient benefit, linear scaling relations fundamentally limit the activity and selectivity of a given class of materials towards a given reaction. Here, we show an example by offering a novel description of the fundamental limits on the activity of CO hydrogenation to methanol; a reaction that offers a sustainable route to obtaining value-added chemicals from syngas. First, we show that there is a strong linear correlation between the formation energy of CO* (where * denotes an adsorbed species) and those of the transition states of a number of elementary steps along the methanol synthesis pathway on these surfaces. Using microkinetic modeling, we cast this information into activity volcano plots with the formation energies of a given transition state and CO* as independent descriptors. This analysis reveals the fundamental limits on activity imposed by the aforementioned linear scaling relations, and invites a vigorous search for novel materials that escape these linear scaling relations as a necessary condition for achieving improved activity towards methanol from CO hydrogenation. Specifically, we point out the transition states H–CO* and CH3O–H* as key transition states to be stabilized independently of CO* for improved activity and selectivity towards methanol synthesis.







Similar content being viewed by others
References
Solomon S, Plattner GK, Knutti R, Friedlingstein P (2009) Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci USA 106(6):1704–1709. https://doi.org/10.1073/pnas.0812721106
Hamelers HVM, Schaetzle O, Paz-Garcia JM, Biesheuvel PM, Buisman CJN (2014) Harvesting energy from CO2 emissions. Environ Sci Technol Lett 1(1):31–35. https://doi.org/10.1021/ez4000059
Bahari NA, Isahak W, Masdar MS, Yaakob Z (2019) Clean hydrogen generation and storage strategies via CO2 utilization into chemicals and fuels: a review. Int J Energy Res 43(10):5128–5150. https://doi.org/10.1002/er.4498
Olah GA (2005) Beyond oil and gas: the methanol economy. Angew Chem Int Ed 44(18):2636–2639. https://doi.org/10.1002/anie.200462121
Olah GA, Goeppert A, Prakash GKS (2009) Chemical recycling off carbon dioxide to methanol and dimethyl ether: from greenhouse gas to renewable, environmentally carbon neutral fuels and synthetic hydrocarbons. J Org Chem 74(2):487–498. https://doi.org/10.1021/jo801260f
Olah GA (2013) Towards oil independence through renewable methanol chemistry. Angew Chem Int Ed 52(1):104–107. https://doi.org/10.1002/anie.201204995
Liu WC, Baek J, Somorjai GA (2018) The methanol economy: methane and carbon dioxide conversion. Top Catal 61(7–8):530–541. https://doi.org/10.1007/s11244-018-0907-4
Cifre PG, Badr O (2007) Renewable hydrogen utilisation for the production of methanol. Energy Convers Manag 48(2):519–527. https://doi.org/10.1016/j.enconman.2006.06.011
Joghee P, Malik JN, Pylypenko S, O'Hayre R (2015) A review on direct methanol fuel cells: in the perspective of energy and sustainability. MRS Energy Sustain. https://doi.org/10.1557/mre.2015.4
Tian P, Wei YX, Ye M, Liu ZM (2015) Methanol to olefins (MTO): from fundamentals to commercialization. ACS Catal 5(3):1922–1938. https://doi.org/10.1021/acscatal.5b00007
Yarulina I, Chowdhury AD, Meirer F, Weckhuysen BM, Gascon J (2018) Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat Catal 1(6):398–411. https://doi.org/10.1038/s41929-018-0078-5
Sheldon D (2017) Methanol production: a technical history a review of the last 100 years of the industrial history of methanol production and a look into the future of the industry. Johnson Matthey Technol Rev 61(3):172–182. https://doi.org/10.1595/205651317x695622
Chanchlani KG, Hudgins RR, Silveston PL (1992) Methanol synthesis from H-2, CO, and CO2 over CU/ZNO catalysts. J Catal 136(1):59–75. https://doi.org/10.1016/0021-9517(92)90106-r
Waugh KC (1992) Mthanol synthesis. Catal Today 15(1):51–75. https://doi.org/10.1016/0920-5861(92)80122-4
Spencer MS (1999) The role of zinc oxide in Cu ZnO catalysts for methanol synthesis and the water-gas shift reaction. Top Catal 8(3–4):259–266. https://doi.org/10.1023/a:1019181715731
Kasatkin I, Kurr P, Kniep B, Trunschke A, Schlogl R (2007) Role of lattice strain and defects in copper particles on the activity of Cu/ZnO/Al2O3 catalysts for methanol synthesis. Angew Chem Int Ed 46(38):7324–7327. https://doi.org/10.1002/anie.200702600
Behrens M (2009) Meso- and nano-structuring of industrial Cu/ZnO/(Al2O3) catalysts. J Catal 267(1):24–29. https://doi.org/10.1016/j.jcat.2009.07.009
Topsoe NY, Topsoe H (1999) On the nature of surface structural changes in Cu ZnO methanol synthesis catalysts. Top Catal 8(3–4):267–270. https://doi.org/10.1023/a:1019133832569
d'Alnoncourt RN, Xia X, Strunk J, Loffler E, Hinrichsen O, Muhler M (2006) The influence of strongly reducing conditions on strong metal-support interactions in Cu/ZnO catalysts used for methanol synthesis. Phys Chem Chem Phys 8(13):1525–1538. https://doi.org/10.1039/b515487a
Grunwaldt JD, Molenbroek AM, Topsoe NY, Topsoe H, Clausen BS (2000) In situ investigations of structural changes in Cu/ZnO catalysts. J Catal 194(2):452–460. https://doi.org/10.1006/jcat.2000.2930
Nakamura I, Nakano H, Fujitani T, Uchijima T, Nakamura J (1998) Evidence for a special formate species adsorbed on the Cu-Zn active site for methanol synthesis. Surf Sci 402(1–3):92–95. https://doi.org/10.1016/s0039-6028(97)00910-2
Nakamura J, Choi Y, Fujitani T (2003) On the issue of the active site and the role of ZnO in Cu/ZnO methanol synthesis catalysts. Top Catal 22(3–4):277–285. https://doi.org/10.1023/a:1023588322846
Kuld S, Thorhauge M, Falsig H, Elkjaer CF, Helveg S, Chorkendorff I, Sehested J (2016) Quantifying the promotion of Cu catalysts by ZnO for methanol synthesis. Science 352(6288):969–974. https://doi.org/10.1126/science.aaf0718
Kuld S, Conradsen C, Moses PG, Chorkendorff I, Sehested J (2014) Quantification of zinc atoms in a surface alloy on copper in an industrial-type methanol synthesis catalyst. Angew Chem Int Ed 53(23):5941–5945. https://doi.org/10.1002/anie.201311073
Kattel S, Ramirez PJ, Chen JG, Rodriguez JA, Liu P (2017) CATALYSIS Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 355(6331):1296. https://doi.org/10.1126/science.aal3573
Behrens M, Studt F, Kasatkin I, Kühl S, Hävecker M, Abild-Pedersen F, Zander S, Girgsdies F, Kurr P, Kniep B-L, Tovar M, Fischer RW, Nørskov JK, Schlögl R (2012) The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336(6083):893–897. https://doi.org/10.1126/science.1219831
Lunkenbein T, Schumann J, Behrens M, Schlogl R, Willinger MG (2015) Formation of a ZnO overlayer in industrial Cu/ZnO/Al2O3 catalysts induced by strong metal-support interactions. Angew Chem Int Ed 54(15):4544–4548. https://doi.org/10.1002/anie.201411581
Schumann J, Eichelbaum M, Lunkenbein T, Thomas N, Galvan MCA, Schlogl R, Behrens M (2015) Promoting strong metal support interaction: doping ZnO for enhanced activity of Cu/ZnO: M (M = Al, Ga, Mg) catalysts. ACS Catal 5(6):3260–3270. https://doi.org/10.1021/acscatal.5b00188
Studt F, Behrens M, Kunkes EL, Thomas N, Zander S, Tarasov A, Schumann J, Frei E, Varley JB, Abild-Pedersen F, Norskov JK, Schlogl R (2015) The mechanism of CO and CO2 hydrogenation to methanol over Cu-based catalysts. ChemCatChem 7(7):1105–1111. https://doi.org/10.1002/cctc.201500123
Yoshihara J, Campbell CT (1996) Methanol synthesis and reverse water-gas shift kinetics over Cu(110) model catalysts: structural sensitivity. J Catal 161(2):776–782. https://doi.org/10.1006/jcat.1996.0240
Rasmussen PB, Holmblad PM, Askgaard T, Ovesen CV, Stoltze P, Norskov JK, Chorkendorff I (1994) Methanol synthesis on CU(100) from a binary gas-mixture of CO2 and H2. Catal Lett 26(3–4):373–381. https://doi.org/10.1007/bf00810611
Szanyi J, Goodman DW (1991) Methanol synthesis on a CU(100) catalyst. Catal Lett 10(5–6):383–390. https://doi.org/10.1007/bf00769173
Gokhale AA, Dumesic JA, Mavrikakis M (2008) On the mechanism of low-temperature water gas shift reaction on copper. J Am Chem Soc 130(4):1402–1414. https://doi.org/10.1021/ja0768237
Studt F, Behrens M, Abild-Pedersen F (2014) Energetics of the water-gas-shift reaction on the active sites of the industrially used Cu/ZnO/Al2O3 catalyst. Catal Lett 144(11):1973–1977. https://doi.org/10.1007/s10562-014-1363-9
Burke K (2012) Perspective on density functional theory. J Chem Phys. https://doi.org/10.1063/1.4704546
Sehested J, Larsen KE, Kustov AL, Frey AM, Johannessen T, Bligaard T, Andersson MP, Norskov JK, Christensen CH (2007) Discovery of technical methanation catalysts based on computational screening. Top Catal 45(1–4):9–13. https://doi.org/10.1007/s11244-007-0232-9
Greeley J, Mavrikakis M (2004) Alloy catalysts designed from first principles. Nat Mater 3(11):810–815. https://doi.org/10.1038/nmat1223
Tameh MS, Dearden AK, Huang C (2018) Accuracy of density functional theory for predicting kinetics of methanol synthesis from CO and CO2 hydrogenation on copper. J Phys Chem C 122(31):17942–17953. https://doi.org/10.1021/acs.jpcc.8b06498
Studt F, Abild-Pedersen F, Wu Q, Jensen AD, Temel B, Grunwaldt J-D, Nørskov JK (2012) CO hydrogenation to methanol on Cu-Ni catalysts: theory and experiment. J Catal 293:51–60. https://doi.org/10.1016/j.jcat.2012.06.004
Studt F, Abild-Pedersen F, Varley JB, Nørskov JK (2013) CO and CO2 hydrogenation to methanol calculated using the BEEF-vdW functional. Catal Lett 143(1):71–73. https://doi.org/10.1007/s10562-012-0947-5
Studt F, Sharafutdinov I, Abild-Pedersen F, Elkjaer CF, Hummelshoj JS, Dahl S, Chorkendorff I, Nørskov JK (2014) Discovery of a Ni-Ga catalyst for carbon dioxide reduction to methanol. Nat Chem 6(4):320–324. https://doi.org/10.1038/nchem.1873
Grabow LC, Mavrikakis M (2011) Mechanism of methanol synthesis on Cu through CO2 and CO hydrogenation. ACS Catal 1(4):365–384. https://doi.org/10.1021/cs200055d
Medford AJ, Lausche AC, Abild-Pedersen F, Temel B, Schjodt NC, Norskov JK, Studt F (2014) Activity and selectivity trends in synthesis gas conversion to higher alcohols. Top Catal 57(1–4):135–142. https://doi.org/10.1007/s11244-013-0169-0
Frei MS, Capdevila-Cortada M, Garcia-Muelas R, Mondelli C, Lopez N, Stewart JA, Ferre DC, Perez-Ramirez J (2018) Mechanism and microkinetics of methanol synthesis via CO2 hydrogenation on indium oxide. J Catal 361:313–321. https://doi.org/10.1016/j.jcat.2018.03.014
Maulana AL, Putra RID, Saputro AG, Agusta MK, Nugraha DHK (2019) DFT and microkinetic investigation of methanol synthesis via CO2 hydrogenation on Ni(111)-based surfaces. Phys Chem Chem Phys 21(36):20276–20286. https://doi.org/10.1039/c9cp02970b
van Rensburg WJ, Petersen MA, Datt MS, van den Berg JA, van Helden P (2015) On the kinetic interpretation of DFT-derived energy profiles: Cu-catalyzed methanol synthesis. Catal Lett 145(2):559–568. https://doi.org/10.1007/s10562-014-1407-1
Tang QL, Zou WT, Huang RK, Wang Q, Duan XX (2015) Effect of the components' interface on the synthesis of methanol over Cu/ZnO from CO2/H-2: a microkinetic analysis based on DFT plus U calculations. Phys Chem Chem Phys 17(11):7317–7333. https://doi.org/10.1039/c4cp05518g
Nie XW, Wang HZ, Janik MJ, Chen YG, Guo XW, Song CS (2017) Mechanistic insight into C-C coupling over Fe-Cu bimetallic catalysts in CO2 hydrogenation. J Phys Chem C 121(24):13164–13174. https://doi.org/10.1021/acs.jpcc.7b02228
Neurock M (1999) First-principles analysis of the hydrogenation of carbon monoxide over palladium. Top Catal 9(3–4):135–152. https://doi.org/10.1023/a:1019179009796
Lam E, Corral-Prez JJ, Larmier K, Noh G, Wolf P, Comas-Vives A, Urakawa A, Copret C (2019) CO2 hydrogenation on Cu/Al2O3: role of metal/support interface in driving activity and selectivity of a bifunctional catalyst. Angew Chem Int Ed 58(39):13989–13996. https://doi.org/10.1002/anie.201908060
Medford AJ, Vojvodic A, Hummelshoj JS, Voss J, Abild-Pedersen F, Studt F, Bligaard T, Nilsson A, Norskov JK (2015) From the Sabatier principle to a predictive theory of transition-metal heterogeneous catalysis. J Catal 328:36–42. https://doi.org/10.1016/j.jcat.2014.12.033
Schumann J, Medford AJ, Yoo JS, Zhao ZJ, Bothra P, Cao A, Studt F, Abild-Pedersen F, Norskov JK (2018) Selectivity of synthesis gas conversion to C2+ oxygenates on fcc(111) transition-metal surfaces. ACS Catal 8(4):3447. https://doi.org/10.1021/acscatal.8b00201
Aresta M, Dibenedetto A (2007) Utilisation of CO2 as a chemical feedstock: opportunities and challenges. Dalton Trans 28:2975–2992. https://doi.org/10.1039/b700658f
Rahimpour MR, Moghtaderi B, Jahanmiri A, Rezaie N (2005) Operability of an industrial methanol synthesis reactor with mixtures of fresh and partially deactivated catalyst. Chem Eng Technol 28(2):226–234. https://doi.org/10.1002/ceat.200407062
Klier K, Chatikavanij V, Herman RG, Simmons GW (1982) Catalytic synthesis of methanol from from CO/H2.4 The effects of carbon-dioxide. J Catal 74(2):343–360. https://doi.org/10.1016/0021-9517(82)90040-9
Fichtl MB, Schlereth D, Jacobsen N, Kasatkin I, Schumann J, Behrens M, Schlogl R, Hinrichsen O (2015) Kinetics of deactivation on Cu/ZnO/Al2O3 methanol synthesis catalysts. Appl Catal A 502:262–270. https://doi.org/10.1016/j.apcata.2015.06.014
Bukhtiyarova M, Lunkenbein T, Kahler K, Schlogl R (2017) Methanol synthesis from industrial CO2 sources: a contribution to chemical energy conversion. Catal Lett 147(2):416–427. https://doi.org/10.1007/s10562-016-1960-x
Sahibzada M, Metcalfe IS, Chadwick D (1998) Methanol synthesis from CO/CO2/H-2 over Cu/ZnO/Al2O3 at differential and finite conversions. J Catal 174(2):111–118. https://doi.org/10.1006/jcat.1998.1964
Yang Y, Mims CA, Mei DH, Peden CHF, Campbell CT (2013) Mechanistic studies of methanol synthesis over Cu from CO/CO2/H-2/H2O mixtures: the source of C in methanol and the role of water. J Catal 298:10–17. https://doi.org/10.1016/j.jcat.2012.10.028
Lee JS, Lee KH, Lee SY, Kim YG (1993) A comparative-study of methanol synthesis from CO2/H-2 and CO/H-2 over a CU/ZNO/AL2O3 catalysts. J Catal 144(2):414–424. https://doi.org/10.1006/jcat.1993.1342
Liu G, Willcox D, Garland M, Kung HH (1985) The role of CO2 in methanol synthesis on CU-ZN oxide: an isotope labeling study. J Catal 96(1):251–260. https://doi.org/10.1016/0021-9517(85)90378-1
Gogate MR (2019) Methanol synthesis revisited: reaction mechanisms in CO/CO2 hydrogenation over Cu/ZnO and DFT analysis. Petrol Sci Technol 37(5):603–610. https://doi.org/10.1080/10916466.2018.1558248
Gunter MM, Ressler T, Bems B, Buscher C, Genger T, Hinrichsen O, Muhler M, Schlogl R (2001) Implication of the microstructure of binary Cu/ZnO catalysts for their catalytic activity in methanol synthesis. Catal Lett 71(1–2):37–44. https://doi.org/10.1023/a:1016696022840
Giannozzi P, Baroni S, Bonini N, Calandra M, Car R, Cavazzoni C, Ceresoli D, Chiarotti GL, Cococcioni M, Dabo I, Dal Corso A, de Gironcoli S, Fabris S, Fratesi G, Gebauer R, Gerstmann U, Gougoussis C, Kokalj A, Lazzeri M, Martin-Samos L, Marzari N, Mauri F, Mazzarello R, Paolini S, Pasquarello A, Paulatto L, Sbraccia C, Scandolo S, Sclauzero G, Seitsonen AP, Smogunov A, Umari P, Wentzcovitch RM (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys. https://doi.org/10.1088/0953-8984/21/39/395502
Wellendorff J, Lundgaard KT, Mogelhoj A, Petzold V, Landis DD, Norskov JK, Bligaard T, Jacobsen KW (2012) Density functionals for surface science: exchange-correlation model development with Bayesian error estimation. Phys Rev B. https://doi.org/10.1103/PhysRevB.85.235149
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188–5192. https://doi.org/10.1103/PhysRevB.13.5188
Henkelman G, Uberuaga BP, Jonsson H (2000) A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J Chem Phys 113(22):9901–9904. https://doi.org/10.1063/1.1329672
Medford AJ, Shi C, Hoffmann MJ, Lausche AC, Fitzgibbon SR, Bligaard T, Norskov JK (2015) CatMAP: a software package for descriptor-based microkinetic mapping of catalytic trends. Catal Lett 145(3):794–807. https://doi.org/10.1007/s10562-015-1495-6
Wang S, Petzold V, Tripkovic V, Kleis J, Howalt JG, Skulason E, Fernandez EM, Hvolbaek B, Jones G, Toftelund A, Falsig H, Bjorketun M, Studt F, Abild-Pedersen F, Rossmeisl J, Norskov JK, Bligaard T (2011) Universal transition state scaling relations for (de)hydrogenation over transition metals. Phys Chem Chem Phys 13(46):20760–20765. https://doi.org/10.1039/c1cp20547a
Abild-Pedersen F, Greeley J, Studt F, Rossmeisl J, Munter TR, Moses PG, Skúlason E, Bligaard T, Nørskov JK (2007) Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.99.016105
Yang NY, Medford AJ, Liu XY, Studt F, Bligaard T, Bent SF, Norskov JK (2016) Intrinsic selectivity and structure sensitivity of rhodium catalysts for C2+ oxygenate production. J Am Chem Soc 138(11):3705–3714. https://doi.org/10.1021/jacs.5b12087
Campbell CT (2001) Finding the rate-determining step in a mechanism: comparing DeDonder relations with the "degree of rate control". J Catal 204(2):520–524. https://doi.org/10.1006/jcat.2001.3396
Foley BL, Bhan A (2020) Degree of rate control and De Donder relations: an interpretation based on transition state theory. J Catal 384:231–251
Stegelmann C, Andreasen A (2011) Finding the key transition states and intermediates controlling net reaction rates and selectivity. Nat Prec. https://doi.org/10.1038/npre.2011.6076.2
Xiao JP, Frauenheim T (2013) Theoretical insights into CO2 activation and reduction on the Ag(111) monolayer supported on a ZnO(000(1)under-bar) substrate. J Phys Chem C 117(4):1804–1808. https://doi.org/10.1021/jp3115868
Chen BWJ, Kirvassilis D, Bai YH, Mavrikakis M (2019) Atomic and molecular adsorption on Ag(111). J Phys Chem C 123(13):7551–7566. https://doi.org/10.1021/acs.jpcc.7b11629
Johansson M, Skulason E, Nielsen G, Murphy S, Nielsen RM, Chorkendorff I (2010) Hydrogen adsorption on palladium and palladium hydride at 1 bar. Surf Sci 604(7–8):718–729. https://doi.org/10.1016/j.susc.2010.01.023
Herron JA, Tonelli S, Mavrikakis M (2012) Atomic and molecular adsorption on Pd(111). Surf Sci 606(21–22):1670–1679. https://doi.org/10.1016/j.susc.2012.07.003
Chen BWJ, Mavrikakis M (2019) Effects of composition and morphology on the hydrogen storage properties of transition metal hydrides: insights from PtPd nanoclusters. Nano Energy. https://doi.org/10.1016/j.nanoen.2019.103858
Schlapbach L, Züttel A (2001) Hydrogen-storage materials for mobile applications. Nature 414(6861):353–358. https://doi.org/10.1038/35104634
Adams BD, Chen AC (2011) The role of palladium in a hydrogen economy. Mater Today 14(6):282–289. https://doi.org/10.1016/s1369-7021(11)70143-2
Jain A, Ong SP, Hautier G, Chen W, Richards WD, Dacek S, Cholia S, Gunter D, Skinner D, Ceder G, Persson KA (2013) Commentary: the materials project: a materials genome approach to accelerating materials innovation. APL Mater. https://doi.org/10.1063/1.4812323
Lin S, Ma JY, Ye XX, Xie DQ, Guo H (2013) CO Hydrogenation on Pd(111): competition between Fischer-Tropsch and oxygenate synthesis pathways. J Phys Chem C 117(28):14667–14676. https://doi.org/10.1021/jp404509v
Lee D, Jung GS, Lee HC, Lee JS (2006) Methanol synthesis over Pd/SiO2 with narrow Pd size distribution prepared by using MCM-41 as a support precursor. Catal Today 111(3–4):373–378. https://doi.org/10.1016/j.cattod.2005.10.062
Perez-Zurita MJ, Cifarelli M, Cubeiro ML, Alvarez J, Goldwasser M, Pietri E, Garcia L, Aboukais A, Lamonier JF (2003) Palladium-based catalysts for the synthesis of alcohols. J Mol Catal A 206(1–2):339–351. https://doi.org/10.1016/s1381-1169(03)00412-6
Matsumura Y, Shen WJ (2003) Methanol decomposition and synthesis over palladium catalysts. Top Catal 22(3–4):271–275. https://doi.org/10.1023/a:1023536306007
Poutsma ML, Elek LF, Ibarbia PA, Risch AP, Rabo JA (1978) Selective formation of methanol from systhesis gas over palladium catalysts. J Catal 52(1):157–168. https://doi.org/10.1016/0021-9517(78)90131-8
Chinchen GC, Denny PJ, Jennings JR, Spencer MS, Waugh KC (1988) Synthesis of methanol. 1. Catalysts and kinetics. Appl Catal 36(1–2):1–65. https://doi.org/10.1016/s0166-9834(00)80103-7
Shen WJ, Okumura M, Matsumura Y, Haruta M (2001) The influence of the support on the activity and selectivity of Pd in CO hydrogenation. Appl Catal A 213(2):225–232. https://doi.org/10.1016/s0926-860x(01)00465-3
Matsumura Y, Shen WJ, Ichihashi Y, Okumura M (2001) Low-temperature methanol synthesis catalyzed over ultrafine palladium particles supported on cerium oxide. J Catal 197(2):267–272. https://doi.org/10.1006/jcat.2000.3094
Hicks RF, Bell AT (1985) Kinetics of mathanol and methane systhesis over PD/SIO2 and PD/LA2O3. J Catal 91(1):104–115. https://doi.org/10.1016/0021-9517(85)90293-3
Berlowitz PJ, Goodman DW (1987) Tha activity of PD(110) for methanol sysnthesis. J Catal 108(2):364–368. https://doi.org/10.1016/0021-9517(87)90185-0
Kim CH, Lee JS, Trimm DL (2003) The preparation and characterisation of Pd-ZnO catalysts for methanol synthesis. Top Catal 22(3–4):319–324. https://doi.org/10.1023/a:1023596524663
Gotti A, Prins R (1996) Effect of metal oxide additives on the CO hydrogenation to methanol over Rh/SiO2 and Pd/SiO2. Catal Lett 37(3–4):143–151. https://doi.org/10.1007/bf00807745
Shen WJ, Ichihashi Y, Okumura M, Matsumura Y (2000) Methanol synthesis from carbon monoxide and hydrogen catalyzed over Pd/CeO2 prepared by the deposition-precipitation method. Catal Lett 64(1):23–25. https://doi.org/10.1023/a:1019070516657
Ulissi ZW, Tang MT, Xiao JP, Liu XY, Torelli DA, Karamad M, Cummins K, Hahn C, Lewis NS, Jaramillo TF, Chan KR, Norskov JK (2017) Machine-learning methods enable exhaustive searches for active bimetallic facets and reveal active site motifs for CO2 reduction. ACS Catal 7(10):6600–6608. https://doi.org/10.1021/acscatal.7b01648
Greeley J (2016) Theoretical heterogeneous catalysis: scaling relationships and computational catalyst design. Annu Rev Chem Biomol Eng 7(7):605–635. https://doi.org/10.1146/annurev-chembioeng-080615-034413
Duyar MS, Tsai C, Snider JL, Singh JA, Gallo A, Yoo JS, Medford AJ, Abild-Pedersen F, Studt F, Kibsgaard J, Bent SF, Norskov JK, Jaramillo TF (2018) A highly active molybdenum phosphide catalyst for methanol synthesis from CO and CO2. Angew Chem Int Ed 57(46):15045–15050. https://doi.org/10.1002/anie.201806583
Sandberg RB, Hansen MH, Norskov JK, Abild-Pedersen F, Bajdich M (2018) Strongly modified scaling of CO hydrogenation in metal supported TiO nanostripes. ACS Catal 8(11):10555–10563. https://doi.org/10.1021/acscatal.8b03327
Jacobsen CJH, Dahl S, Clausen BS, Bahn S, Logadottir A, Nørskov JK (2001) Catalyst design by interpolation in the periodic table: bimetallic ammonia synthesis catalysts. J Am Chem Soc 123(34):8404–8405. https://doi.org/10.1021/ja010963d
Hansen HA, Shi C, Lausche AC, Peterson AA, Norskov JK (2016) Bifunctional alloys for the electroreduction of CO2 and CO. Phys Chem Chem Phys 18(13):9194–9201. https://doi.org/10.1039/c5cp07717f
Pedersen JK, Batchelor TAA, Bagger A, Rossmeisl J (2020) High-entropy alloys as catalysts for the CO2 and CO reduction reactions. ACS Catal 10(3):2169–2176. https://doi.org/10.1021/acscatal.9b04343
Greeley J, Mavrikakis M (2006) Near-surface alloys for hydrogen fuel cell applications. Catal Today 111(1–2):52–58. https://doi.org/10.1016/j.cattod.2005.10.009
Darby MT, Reocreux R, Sykes ECH, Michaelides A, Stamatakis M (2018) Elucidating the stability and reactivity of surface intermediates on single-atom alloy catalysts. ACS Catal 8(6):5038–5050. https://doi.org/10.1021/acscatal.8b00881
Darby MT, Stamatakis M, Michaelides A, Sykes ECH (2018) Lonely atoms with special gifts: breaking linear scaling relationships in heterogeneous catalysis with single-atom alloys. J Phys Chem Lett 9(18):5636–5646. https://doi.org/10.1021/acs.jpclett.8b01888
Marcinkowski MD, Darby MT, Liu JL, Wimble JM, Lucci FR, Lee S, Michaelides A, Flytzani-Stephanopoulos M, Stamatakis M, Sykes ECH (2018) Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C-H activation. Nat Chem 10(3):325–332. https://doi.org/10.1038/nchem.2915
Liu JL, Lucci FR, Yang M, Lee S, Marcinkowski MD, Therrien AJ, Williams CT, Sykes ECH, Flytzani-Stephanopoulos M (2016) Tackling CO poisoning with single-atom alloy aatalysts. J Am Chem Soc 138(20):6396–6399. https://doi.org/10.1021/jacs.6b03339
Brankovic SR, Wang JX, Adžić RR (2001) Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf Sci 474(1–3):L173–L179. https://doi.org/10.1016/s0039-6028(00)01103-1
Yang L, Vukmirovic MB, Su D, Sasaki K, Herron JA, Mavrikakis M, Liao S, Adzic RR (2013) Tuning the catalytic activity of Ru@Pt core-shell nanoparticles for the oxygen reduction reaction by varying the shell thickness. J Phys Chem C 117(4):1748–1753. https://doi.org/10.1021/jp309990e
Sasaki K, Naohara H, Cai Y, Choi YM, Liu P, Vukmirovic MB, Wang JX, Adzic RR (2010) Core-protected platinum monolayer shell high-stability electrocatalysts for fuel-cell cathodes. Angew Chem Int Ed 49(46):8602–8607. https://doi.org/10.1002/anie.201004287
Semagina N, Kiwi-Minsker L (2009) Recent advances in the liquid-phase synthesis of metal nanostructures with controlled shape and size for catalysis. Catal Rev 51(2):147–217. https://doi.org/10.1080/01614940802480379
Xia YN, Xia XH, Wang Y, Xie SF (2013) Shape-controlled synthesis of metal nanocrystals. MRS Bull 38(4):335–344. https://doi.org/10.1557/mrs.2013.84
Ruditskiy A, Choi S-I, Peng H-C, Xia Y (2014) Shape-controlled metal nanocrystals for catalytic applications. MRS Bull 39(8):727–737. https://doi.org/10.1557/mrs.2014.167
Ruditskiy A, Peng HC, Xia YN (2016) Shape-controlled metal nanocrystals for heterogeneous catalysis. Annu Rev Chem Biomol Eng 7(7):327–348. https://doi.org/10.1146/annurev-chembioeng-080615-034503
Xia YN, Xia XH, Peng HC (2015) Shape-controlled synthesis of colloidal metal nanocrystals: thermodynamic versus kinetic products. J Am Chem Soc 137(25):7947–7966. https://doi.org/10.1021/jacs.5b04641
Nerlov J, Chorkendorff I (1998) Promotion through gas phase induced surface segregation: methanol synthesis from CO, CO2 and H-2 over Ni/Cu(100). Catal Lett 54(4):171–176. https://doi.org/10.1023/a:1019033517855
Nerlov J, Chorkendorff I (1999) Methanol synthesis from CO2, CO, and H-2 over Cu(100) and Ni/Cu(100). J Catal 181(2):271–279. https://doi.org/10.1006/jcat.1998.2301
Nerlov J, Sckerl S, Wambach J, Chorkendorff I (2000) Methanol synthesis from CO2, CO and H-2 over Cu(100) and Cu(100) modified by Ni and Co. Appl Catal A 191(1–2):97–109. https://doi.org/10.1016/s0926-860x(99)00311-7
Zegkinoglou I, Pielsticker L, Han ZK, Divins NJ, Kordus D, Chen YT, Escudero C, Perez-Dieste V, Zhu BE, Gao Y, Cuenya BR (2019) Surface segregation in CuNi nanoparticle catalysts during CO2 hydrogenation: the role of CO in the reactant mixture. J Phys Chem C 123(13):8421–8428. https://doi.org/10.1021/acs.jpcc.8b09912
Darby MT, Sykes ECH, Michaelides A, Stamatakis M (2018) Carbon monoxide poisoning resistance and structural stability of single atom alloys. Top Catal 61(5–6):428–438. https://doi.org/10.1007/s11244-017-0882-1
Papanikolaou KG, Darby MT, Stamatakis M (2019) CO-induced aggregation and segregation of highly dilute alloys: a density functional theory study. J Phys Chem C 123(14):9128–9138. https://doi.org/10.1021/acs.jpcc.9b00649
Tran K, Palizhati A, Back S, Ulissi ZW (2018) Dynamic workflows for routine materials discovery in surface science. J Chem Inf Model 58(12):2392–2400. https://doi.org/10.1021/acs.jcim.8b00386
Ulissi ZW, Medford AJ, Bligaard T, Norskov JK (2017) To address surface reaction network complexity using scaling relations machine learning and DFT calculations. Nat Commun. https://doi.org/10.1038/ncomms14621
Ward L, Aykol M, Blaiszik B, Foster I, Meredig B, Saal J, Suram S (2018) Strategies for accelerating the adoption of materials informatics. MRS Bull 43(9):683–689. https://doi.org/10.1557/mrs.2018.204
Schmidt J, Marques MRG, Botti S, Marques MAL (2019) Recent advances and applications of machine learning in solid-state materials science. NPJ Comput Mater. https://doi.org/10.1038/s41524-019-0221-0
Lamoureux PS, Winther KT, Torres JAG, Streibel V, Zhao M, Bajdich M, Abild-Pedersen F, Bligaard T (2019) Machine learning for computational heterogeneous catalysis. ChemCatChem 11(16):3579–3599. https://doi.org/10.1002/cctc.201900595
Lin LS (2015) Materials databases infrastructure constructed by first principles calculations: a review. Mater Perform Charact 4(1):148–169. https://doi.org/10.1520/mpc20150014
Ma XF, Xin HL (2017) Orbitalwise coordination number for predicting adsorption properties of metal nanocatalysts. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.118.036101
Calle-Vallejo F, Martinez JI, Garcia-Lastra JM, Sautet P, Loffreda D (2014) Fast prediction of adsorption properties for platinum nanocatalysts with generalized coordination numbers. Angew Chem Int Ed 53(32):8316–8319. https://doi.org/10.1002/anie.201402958
Calle-Vallejo F, Bandarenka AS (2018) Enabling generalized coordination numbers to describe strain effects. Chemsuschem 11(11):1824–1828. https://doi.org/10.1002/cssc.201800569
Choksi TS, Roling LT, Streibel V, Abild-Pedersen F (2019) Predicting adsorption properties of catalytic descriptors on bimetallic nanoalloys with site-specific precision. J Phys Chem Lett 10(8):1852–1859. https://doi.org/10.1021/acs.jpclett.9b00475
Roling LT, Li L, Abild-Pedersen F (2017) Configurational energies of nanoparticles based on metal-metal coordination. J Phys Chem C 121(41):23002–23010. https://doi.org/10.1021/acs.jpcc.7b08438
Roling LT, Abild-Pedersen F (2018) Structure-sensitive scaling relations: adsorption energies from surface site stability. ChemCatChem 10(7):1643–1650. https://doi.org/10.1002/cctc.201701841
Roling LT, Choksi TS, Abild-Pedersen F (2019) A coordination-based model for transition metal alloy nanoparticles. Nanoscale 11(10):4438–4452. https://doi.org/10.1039/c9nr00959k
Andersen M, Levchenko SV, Scheffler M, Reuter K (2019) Beyond scaling relations for the description of catalytic materials. ACS Catal 9(4):2752–2759. https://doi.org/10.1021/acscatal.8b04478
Toyao T, Suzuki K, Kikuchi S, Takakusagi S, Shimizu K, Takigawa I (2018) Toward effective utilization of methane: machine learning prediction of adsorption energies on metal alloys. J Phys Chem C 122(15):8315–8326. https://doi.org/10.1021/acs.jpcc.7b12670
Tran K, Ulissi ZW (2018) Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H-2 evolution. Nat Catal 1(9):696–703. https://doi.org/10.1038/s41929-018-0142-1
Li Z, Wang SW, Chin WS, Achenie LE, Xin HL (2017) High-throughput screening of bimetallic catalysts enabled by machine learning. J Mater Chem A 5(46):24131–24138. https://doi.org/10.1039/c7ta01812f
Back S, Tran K, Ulissi ZW (2019) Toward a design of active oxygen evolution catalysts: insights from automated density functional theory calculations and machine learning. ACS Catal 9(9):7651–7659. https://doi.org/10.1021/acscatal.9b02416
Singh AR, Rohr BA, Gauthier JA, Norskov JK (2019) Predicting chemical reaction barriers with a machine learning model. Catal Lett 149(9):2347–2354. https://doi.org/10.1007/s10562-019-02705-x
Peterson AA (2016) Acceleration of saddle-point searches with machine learning. J Chem Phys. https://doi.org/10.1063/1.4960708
Torres JAG, Jennings PC, Hansen MH, Boes JR, Bligaard T (2019) Low-scaling algorithm for nudged elastic band calculations using a surrogate machine learning model. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.122.156001
Acknowledgements
The authors acknowledge support through V-Sustain: The VILLUM Centre for the Science of Sustainable Fuels and Chemicals (#20886) from VILLUM FONDEN.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elnabawy, A.O., Schumann, J., Bothra, P. et al. The Challenge of CO Hydrogenation to Methanol: Fundamental Limitations Imposed by Linear Scaling Relations. Top Catal 63, 635–648 (2020). https://doi.org/10.1007/s11244-020-01283-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-020-01283-2