Abstract
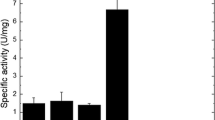
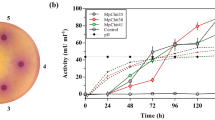
A recombinant strain producing a complex of extracellular enzymes including chitinase from Myceliophtora thermophila was created based on the fungus Penicillium verruculosum. The activity of the enzyme preparations obtained from the cultural fluid of the producer strain was 0.55, 0.53, and 0.66 U/mg protein with chitin and chitosans with the molecular weight of 200 and 1000 kDa, respectively. The temperature optimum for the recombinant chitinase was 52-65°C; the pH optimum was 4.5-6.2, which corresponded to the published data for this class of the enzymes. The content of heterologous chitinase in the obtained enzyme preparations was 47% of total protein content in the cultural fluid. Enzyme preparations produced by the recombinant P. verruculosum XT403 strain and containing heterologous chitinase were able to degrade the mycelium of micromycetes, including phytopathogenic ones, and were very efficient in the bioconversion of microbiological industry waste.





Similar content being viewed by others
Abbreviations
- CBHI:
-
cellobiohydrolase I
- CF:
-
cultural fluid
- CHT:
-
chitinase
- DD:
-
degree of deacetylation
- EG:
-
endoglucanase
- EP:
-
enzyme preparation
- XYL:
-
xylanase
REFERENCES
Melent’ev, A. I., and Aktuganov, G. E. (2013) in Enzymes of Degradation of Chitin and Chitosan. Chitosan [in Russian] (Skryabin, G. K., Mikhailov, C. N., and Varlamov, V. P., eds.) Center “Bioengineering”, Russian Academy of Sciences, Moscow, pp. 71-114.
Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activity and Applications (2011) (Kim, S.-K., ed.) CRC Press, Taylor and Francis Group, Boca Raton, London, N. Y., doi: 10.1201/EBK1439816035.
Berini, F., Katz, C., Gruzdev, N., Casartelli, M., Tettamanti, G., and Marinelli, F. (2018) Microbial and viral chitinases: attractive biopesticides for integrated pest management, Biotechnol. Adv., 36, 818-828, doi: 10.1016/j.biotechadv.2018.01.002.
Vijayakumar, N., and Alagar, S. (2017) Consequence of chitinase from Trichoderma viride integrated feed on digestive enzymes in Corcyra cephalonica (Stainton) and antimicrobial potential, Biosci. Biotech. Res. Asia, 14, 513-519, doi: 10.13005/bbra/2473.
Sakai, K., Uchiyama, T., Matahira, Y., and Nanjo, F. (1991) Immobilization of chitinolytic enzymes and continuous production of N-acetyl D-glucosamine with the immobilized enzymes, J. Ferment. Bioeng., 72, 168-172, doi: 10.1016/0922-338X(91)90211-X.
Saschiwa, H., Fujishima, S., and Yamano, N. (2002) Production of N-acetyl D-glucosamine from alpha-chitin by crude enzymes from Aeromonas hydrophila H-2330, Carbohydr. Res., 337, 761-763, doi: 10.1016/s0008-6215(02)00034-4.
Jain, T., Kumar, H., and Dutta, P. K. (2016) in Chitin and Chitosan for Regenerative Medicine (Dutta, P. K., ed.) Springer India, Bangalore, Mumbai, New Delhi, India, pp. 279-295.
Wang, S.-L., Liang, T.-W., and Yen, Y.-H. (2011) Bioconversion of chitin-containing wastes for the production of enzymes and bioactive materials, Carbohydr. Pol., 84, 732-742, doi: 10.1016/j.carbpol.2010.06.022.
Thadathil, N., and Velappan, S. P. (2014) Recent developments in chitosanase research and its biotechnological applications: a review, Food Chem., 150, 392-399, doi: 10.1016/j.foodchem.2013.10.083.
Sakai, K., Yokota, A., Kurokawa, H., Wakayama, M., and Moriguchi, M. (1998) Purification and characterization of three thermostable endochitinases of a noble Bacillus strain, MH-1, isolated from chitin-containing compost, Appl. Environ. Microbiol., 64, 3397-3402, doi: 10.1128/AEM.64.9.3397-3402.1998.
Revah-Moiseev, S., and Carroad, P. A. (1981) Conversion of the enzymatic hydrolysate of shellfish waste chitin to single-cell protein, Biotechnol. Bioeng., 23, 1067-1078, doi: 10.1002/bit.260230514.
Dahia, N., Tiwari, R., Tiwari, R. P., and Hoondal, G. S. (2005) Production of an antifungal chitinase from Enterobacter sp. NRG4 and its application in protoplast production, World J. Microbiol. Biotechnol., 21, 1611-1615, doi: 10.1007/s11274-005-8343-6.
Johnson, E. A., Villa, T. G., Lewis, M. J., and Phaff, H. J. (1979) Lysis of the cell wall of yeast Phaffia rhodozyme by lytic complex from Bacillus circulans WL-12, J. Appl. Biochem., 1, 273-282.
Biotransformation of Waste Biomass into High Value Biochemicals (2014) (Brar, S. K., Dhillon, G. S., and Soccol, C., eds.) Springer Verlag, N. Y., p. 504.
Kamzolkina, O. V., and Dunaevsky, Y. E. (2017) Biology of a Fungal Cell. A Handbook [in Russian], 2nd Edn., Fellowship of Scientific Publications KMK, Moscow.
Kuranda, M. J., and Robbins, P. W. (1991) Chitinase is required for cell separation during growth of Saccharomyces cerevisiae, J. Biol. Chem., 266, 19707-19758.
Kurchenko, V. P., Buga, S. V., Petrashkevich, H. V., Butkevich, T. V., Vetoshkin, A. A., Demchenkov, E. L., Lodygin, A. D., Zueva, O. Y., Varlamov, V. P., and Borodin, O. I. (2016) Technological bases of obtaining Chitin and chitosan from insects, Trudy BGU, 11, 110-126.
Sereda, A. S., Velikortskaya, I. A., Osipov, D. O., Matys, V. Y., Bubnova, T. V., Nemashkalov, V. A., Sinitsyna, O. A., Rozhkova, A. M., Tsurikova, N. V., and Sinitsyn, A. P. (2018) Enzyme complexes for destruction of the cell wall of mycelial fungi – producers industrial enzymes, Izv. of Res. Center of Ufa, Russian Academy of Sciences, 3, 31-35.
Sinitsyn, A. P., Okunev, O. N., Chernoglazov, V. M., Sinitsyna, O. A., and Popov, V. O. The RF Patent No. 2361918, published 20.07.2009.
Sinitsyn, A. P., Rozhkova , A. M., Sinitsyna, O. A., Fedorova, E. A., Okunev, O. N., Bekkarevich, A. O., Sokolova, L. M., Matys, V. Yu., Koshelev, A. V., Vinetskiy, Yu. P., Chernoglazov, V. M., and Zorov, I. N. The RF Patent No. 2378372, published 10.01.2010.
Sinitsyn, A. P., Korotkova, O. G., Rubtsova, E. A., Sinitsyna, O. A., Kondrat’eva, E. G., Sereda, A. S., Zorov, I. N., and Rozhkova, A. M. (2019) Constructing recombinant producers of enzyme preparations for production of fodder with the expression system based on the fungus Penicillium verruculosum, Biotechnologiya, 35, 6-14, doi: 10.21519/0234-2758-2019-35-4-6-14.
Bukhtoyarov, F. E., Ustinov, B. B., Salanovich, T. N., Antonov, A. I., Gusakov, A. V., Okunev, O. N., and Sinitsyn, A. P. (2004) Cellulase complex of the fungus Chrysosporium lucknowense: isolation and characteristics of endoglucanases and cellobiohydrolases, Biochemistry (Moscow), 69, 542-551.
Merzlov, D. A., Zorov, I. N., Dotsenko, G. S., Denisenko, Y. A., Rozhkova, A. M., Satrutdinov, A. D., Rubtsova, E. A., Kondrat’eva, E. G., and Sinitsyn, A. P. (2015) Properties of enzyme preparations and homogenous enzymes – endoglucanase EG2 Penicillium verruculosum and endoglucanase LAM Myceliophtora thermophila, Biochemistry (Moscow), 80, 473-482, doi: 10/1134/S006297915040112.
Semenova, M. V., Gusakov, A. V., Volkov, P. V., Matys, V. Yu., Nemashkalov, V. A., Telitsyn, V. D., Rozhkova, A. M., and Sinitsyn, A. P. (2019) Enhancement of the enzymatic cellulose saccharification by Penicillium verruculosum multienzyme cocktails containing homologously overexpressed lytic polysaccharide monooxygenase, Mol. Biol. Rep., 46, 2363-2370, doi: 10.1007/s11033-019-04693-y.
Aslanidis, C., and de Jong, J. P. (1990) Ligation-independent cloning of PCR products (LIC-PCR), Nucleic Acids Res., 18, 6069-6075, doi: 10.1093/nar/18.20.6069.
Nelson, N. A. (1944) Photometric adoption of the Somogyi method for the determination of glucose, J. Biol. Chem., 153, 375-379.
Somogyi, M. (1952) Notes on sugar determination, J. Biol. Chem., 195, 19-23.
Sinitsyna, O. A., Bukhtoyarov, F. E., Gusakov, A. V., Okunev, O. N., Bekarevich, A. O., Vinetsky, Y. P., and Sinitsyn, A. P. (2003) Isolation and properties of major components of Penicillium canescens extracellular enzyme complex, Biochemistry (Moscow), 68, 1200-1209, doi: 10.1023/b:biry.0000009134.48246.7e.
Zorov, I. N., Dubasova, M. Y., Sinitsyn, A. P., Gusakov, A. V., Mytchenko, A. A., Baraznenok, V. A., Gutierrez, B., and Popova, N. N. (1997) Application of the bicinchininic method of assay for the reducing sugars to determine carboxymethylcellulase activity of cellulases using a microplate reader, Biochemistry (Moscow), 62, 704-709, doi: 10.1134/S0006297910010062.
Sinitsyn, A. P., Chernoglazov, V. M., and Gusakov, A. V. (1993) Methods of Study and Properties of Cellulolytic Enzymes. Series Biotechnologiya, 25 (Summation of Science and Technique, Academy of Sciences of USSR), VINITI, Moscow, [in Russian].
Decleire, M., De Cat, W., and Tang, V. H. (1996) in Chitin Enzymology (Muzzarelli, R. A. A., ed.) Atec Edizioni, Glottamare, Italy, pp. 165-169.
Peterson, G. L. (1977) Review of the Folin phenol protein quantitation method of Lowry, Rosebrough, Farr and Randall, Anal. Biochem., 100, 201-220, doi: 10.1016/0003-2697(79)90222-7.
Aleksenko, A. Y., Makarova, N. A., Nikolaev, I. V., and Clutterbuck, A. J. (1995) Integrative and replicative transformation of Penicillium canescens with a heterologous nitrate-reductase gene, Curr. Genet., 28, 474-478, doi: 10.1007/BF00310818.
Chulkin, A. M., Kislitsin, V. Y., Zorov, I. N., Sinitsyn, A. P., and Rozhkova, A. M. (2019) Determination of the copy number of the purpose genes of carbohydrases in recombinant strain of the fungus Penicillium verruculosum, Biotekhnologiya, 35, 51-57, doi: 10.21519/0234-2758-2019-35-5-51-57.
Acknowledgements
We used the equipment of the Industrial Biotechnologies Center for Collective Use of the Federal Research Center of Biotechnology, Russian Academy of Sciences.
Funding
This work was supported by the Ministry of Science and Higher Education (unique project identifier RFMEFI61620X0128).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain description of studies with the involvement of humans or animals performed by any of the authors. The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sinitsyna, O., Rubtsova, E., Sinelnikov, I. et al. Creation of Chitinase Producer and Disruption of Micromycete Cell Wall with the Obtained Enzyme Preparation. Biochemistry Moscow 85, 717–724 (2020). https://doi.org/10.1134/S0006297920060097
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920060097