Abstract
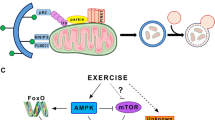
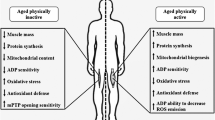
Up to now numerous studies in the field of gerontology have been published. Nevertheless, a well-known food restriction remains the most reliable and efficient way of lifespan extension. Physical activity is also a well-documented anti-aging intervention being especially efficient in slowing down the age-associated decline of skeletal muscle mass. In this review we focus on the molecular mechanisms of the effect of physical exercise on muscle tissues. We also discuss the possibilities of pharmacological extension of this effect to the rest of the tissues. During the exercise, the level of ATP decreases triggering activation of AMP-dependent protein kinase (AMPK). This kinase stimulates antioxidant potential of the cells and their mitochondrial respiratory capacity. The exercise also induces mild oxidative stress, which, in turn, mediates the stimulation via hormetic response. Furthermore, during the exercise cells generate activators of mammalian target of rapamycin (mTOR). The intracellular ATP level increases during the rest periods between exercises thus promoting mTOR activation. Therefore, regular exercise intermittently activates anti-oxidant defenses and mitochondrial biogenesis (via AMPK and the hormetic response) of the muscle tissue, as well as its proliferative potential (via mTOR), which, in turn, impedes the age-dependent muscle atrophy. Thus, the intermittent treatment with activators of (i) AMPK combined with the inducers of hormetic response and of (ii) mTOR might partly mimic the effects of physical exercise. Importantly, pharmacological activation of AMPK takes place in the absence of ATP level decrease. The use of uncouplers of respiration and oxidative phosphorylation at the phase of AMPK activation could also prevent negative consequences of the cellular hyper-energization. It is believed that the decline of both antioxidant and proliferative potentials of the cells causes the age-dependent decline of multiple tissues, rather than only the muscular one. We argue that the approach above is applicable for the majority of tissues in an organism.

Similar content being viewed by others
Abbreviations
- AICAR:
-
5-aminoimidazole-4-carboxamide ribonucleotide
- AMPK:
-
AMP-dependent protein kinase
- GH:
-
growth hormone
- IGF1:
-
insulin-like growth factor 1
- MGF:
-
muscle growth factor
- mTOR:
-
mammalian target of rapamycin
- ROS:
-
reactive oxygen species
REFERENCES
Qian, M., and Liu, B. (2018) Pharmaceutical intervention of aging, Adv. Exp. Med. Biol., 1086, 235-254, doi: 10.1007/978-981-13-1117-8_15.
Mercken, E. M., Carboneau, B. A., Krzysik-Walker, S. M., and de Cabo, R. (2012) Of mice and men: the benefits of caloric restriction, exercise, and mimetics, Ageing Res. Rev., 11, 390-398, doi: 10.1016/j.arr.2011.11.005.
Palliyaguru, D. L., Moats, J. M., Di Germanio, C., Bernier, M., and de Cabo, R. (2019) Frailty index as a biomarker of lifespan and healthspan: focus on pharmacological interventions, Mech. Ageing Dev., 180, 42-38, doi: 10.1016/j.mad.2019.03.005.
Martel, J., Ojcius, D. M., Ko, Y.-F., Chang, C.-J., and Young, J. D. (2019) Antiaging effects of bioactive molecules isolated from plants and fungi, Med. Res. Rev., 39, 1515-1552, doi: 10.1002/med.21559.
Fontana, L., Partridge, L., and Longo, V. D. (2010) Extending healthy life span – from yeast to humans, Science, 328, 321-326, doi: 10.1126/science.1172539.
Mulvey, L., Sinclair, A., and Selman, C. (2014) Lifespan modulation in mice and the confounding effects of genetic background, J. Genet. Genomics, 41, 497-503, doi: 10.1016/j.jgg.2014.06.002.
Holloszy, J. O. (1998) Longevity of exercising male rats: effect of an antioxidant supplemented diet, Mech. Ageing Dev., 100, 211-219, doi: 10.1016/s0047-6374(97)00140-1.
Prado, C. M., Purcell, S. A., Alish, C., Pereira, S. L., Deutz, N. E., Heyland, D. K., Goodpaster, B. H., Tappenden, K. A., and Heymsfield, S. B. (2018) Implications of low muscle mass across the continuum of care: a narrative review, Ann. Med., 50, 675-693, doi: 10.1080/07853890.2018.1511918.
Distefano, G., and Goodpaster, B. H. (2018) Effects of exercise and aging on skeletal muscle, Cold Spring Harb. Perspect. Med., 8, doi: 10.1101/cshperspect.a029785.
Hernández-Álvarez, D., Mena-Montes, B., Toledo-Pérez, R., PedrazaVázquez, G., López-Cervantes, S. P., Morales-Salazar, A., Hernández-Cruz, E., Lazzarini-Lechuga, R., Vázquez-Cárdenas, R. R., Vilchis-DeLaRosa, S., Posadas-Rodríguez, P., Santín-Márquez, R., Rosas-Carrasco, O., Ibañez-Contreras, A., Alarcón-Aguilar, A., López-Díazguerrero, N. E., Luna-López, A., and Königsberg, M. (2019) Long-term moderate exercise combined with metformin treatment induces an hormetic response that prevents strength and muscle mass loss in old female wistar rats, Oxid. Med. Cell. Longev., 2019, 3428543, doi: 10.1155/2019/3428543.
Li, F.-H., Sun, L., Zhu, M., Li, T., Gao, H.-E., Wu, D.-S., Zhu, L., Duan, R., and Liu, T. C. (2018) Beneficial alterations in body composition, physical performance, oxidative stress, inflammatory markers, and adipocytokines induced by long-term high-intensity interval training in an aged rat model, Exp. Gerontol., 113, 150-162, doi: 10.1016/j.exger.2018.10.006.
Klimova, B., Novotny, M., and Kuca, K. (2018) Anti-aging drugs – prospect of longer life? Curr. Med. Chem., 25, 1946-1953, doi: 10.2174/0929867325666171129215251.
Carmona, J. J., and Michan, S. (2016) Biology of healthy aging and longevity, Rev. Invest. Clin., 68, 7-16.
Martinez-Lopez, N., Tarabra, E., Toledo, M., Garcia-Macia, M., Sahu, S., Coletto, L., Batista-Gonzalez, A., Barzilai, N., Pessin, J. E., Schwartz, G. J., Kersten, S., and Singh, R. (2017) System-wide benefits of intermeal fasting by autophagy, Cell Metab., 26, 856-871, doi: 10.1016/j.cmet.2017.09.020.
Jamshed, H., Beyl, R. A., Della Manna, D. L., Yang, E. S., Ravussin, E., and Peterson, C. M. (2019) Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans, Nutrients, 11, doi: 10.3390/nu11061234.
Hawley, J. A., and Holloszy, J. O. (2009) Exercise: it’s the real thing! Nutr. Rev., 67, 172-178, doi: 10.1111/j.1753-4887.2009.00185.x.
Knorre, D. A., and Severin, F. F. (2016) Uncouplers of oxidation and phosphorylation as antiaging compounds, Biochemistry (Moscow), 81, 1438-1444, doi: 10.1134/S0006297916120051.
Espinosa, A., Henríquez-Olguín, C., and Jaimovich, E. (2016) Reactive oxygen species and calcium signals in skeletal muscle: a crosstalk involved in both normal signaling and disease, Cell Calcium, 60, 172-179, doi: 10.1016/j.ceca.2016.02.010.
Ferreira, L. F., and Laitano, O. (2016) Regulation of NADPH oxidases in skeletal muscle, Free Radic. Biol. Med., 98, 18-28, doi: 10.1016/j.freeradbiomed.2016.05.011.
Ji, L. L., Kang, C., and Zhang, Y. (2016) Exercise-induced hormesis and skeletal muscle health, Free Radic. Biol. Med., 98, 113-22, doi: 10.1016/j.freeradbiomed.2016.02.025.
Musci, R. V., Hamilton, K. L., and Linden, M. A. (2019) Exercise-induced mitohormesis for the maintenance of skeletal muscle and healthspan extension, Sports (Basel), 7, doi: 10.3390/sports7070170.
Webb, R., Hughes, M. G., Thomas, A. W., and Morris, K. (2017) The ability of exercise-associated oxidative stress to trigger redox-sensitive signalling responses, Antioxidants (Basel), 6, doi: 10.3390/antiox6030063.
Merry, T. L., and Ristow, M. (2016) Mitohormesis in exercise training, Free Radic. Biol. Med., 98, 123-130, doi: 10.1016/j.freeradbiomed.2015.11.032.
Ferrer, A., Caelles, C., Massot, N., and Hegardt, F. G. (1985) Activation of rat liver cytosolic 3-hydroxy-3-methylglutaryl coenzyme A reductase kinase by adenosine 5′-monophosphate, Biochem. Biophys. Res. Commun., 132, 497-504, doi: 10.1016/0006-291x(85)91161-1.
Carling, D., Clarke, P. R., Zammit, V. A., and Hardie, D. G. (1989) Purification and characterization of the AMP-activated protein kinase. Copurification of acetyl-CoA carboxylase kinase and 3-hydroxy-3-methylglutaryl-CoA reductase kinase activities, Eur. J. Biochem., 186, 129-136, doi: 10.1111/j.1432-1033.1989.tb15186.x.
Hardie, D. G., and Carling, D. (1997) The AMP-activated protein kinase – fuel gauge of the mammalian cell? Eur. J. Biochem., 246, 259-273, doi: 10.1111/j.1432-1033.1997.00259.x.
Hardie, D. G. (2011) Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism, Proc. Nutr. Soc., 70, 92-99, doi: 10.1017/S0029665110003915.
Guerrieri, D., Moon, H. Y., and van Praag, H. (2017) Exercise in a pill: the latest on exercise-mimetics, Brain Plast., 2, 153-169, doi: 10.3233/BPL-160043.
Vilchinskaya, N. A., Krivoi, I. I., and Shenkman, B. S. (2018) AMP-activated protein kinase as a key trigger for the disuse-induced skeletal muscle remodeling, Int. J. Mol. Sci., 19, doi: 10.3390/ijms19113558.
Bodur, C., Karakas, B., Timucin, A. C., Tezil, T., and Basaga, H. (2016) AMP-activated protein kinase couples 3-bromopyruvate-induced energy depletion to apoptosis via activation of FoxO3a and upregulation of proapoptotic Bcl-2 proteins, Mol. Carcinogen., 55, 1584-1597, doi: 10.1002/mc.22411.
Shin, S., Buel, G. R., Wolgamott, L., Plas, D. R., Asara, J. M., Blenis, J., and Yoon, S. O. (2015) ERK2 mediates metabolic stress response to regulate cell fate, Mol. Cell, 59, 382-398, doi: 10.1016/j.molcel.2015.06.020.
Green, D. R., Galluzzi, L., and Kroemer, G. (2014) Cell biology. Metabolic control of cell death, Science, 345, 1250256, doi: 10.1126/science.1250256.
Su, K.-H., Dai, S., Tang, Z., Xu, M., and Dai, C. (2019) Heat shock factor 1 is a direct antagonist of AMP-activated protein kinase, Mol. Cell, 76, 546-561, doi: 10.1016/j.molcel.2019.08.021.
Sharples, A. P., Hughes, D. C., Deane, C. S., Saini, A., Selman, C., and Stewart, C. E. (2015) Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake, Aging Cell, 14, 511-523, doi: 10.1111/acel.12342.
Thomson, D. M. (2018) The role of AMPK in the regulation of skeletal muscle size, hypertrophy, and regeneration, Int. J. Mol. Sci., 19, doi: 10.3390/ijms19103125.
Ost, M., Coleman, V., Kasch, J., and Klaus, S. (2016) Regulation of myokine expression: role of exercise and cellular stress, Free Radic. Biol. Med., 98, 78-89, doi: 10.1016/j.freeradbiomed.2016.02.018.
Eckel, J. (2019) Myokines in metabolic homeostasis and diabetes, Diabetologia, 62, 1523-1538, doi: 10.1007/s00125-019-4927-9.
Graf, C., and Ferrari, N. (2019) Metabolic health – the role of adipo-myokines, Int. J. Mol. Sci., 20, doi: 10.3390/ijms20246159.
Zhang, M., Pan, K., Liu, Q., Zhou, X., Jiang, T., and Li, Y. (2016) Growth differentiation factor 15 may protect the myocardium from no-reflow by inhibiting the inflammatory-like response that predominantly involves neutrophil infiltration, Mol. Med. Rep., 13, 623-362, doi: 10.3892/mmr.2015.4573.
Zhang, Y., Moszczynski, L. A., Liu, Q., Jiang, J., Zhao, D., Quan, D., Mele, T., McAlister, V., Jevnikar, A., Baek, S. J., Liu, K., and Zheng, X. (2017) Over-expression of growth differentiation factor 15 (GDF15) preventing cold ischemia reperfusion (I/R) injury in heart transplantation through Foxo3a signaling, Oncotarget, 8, 36531-3644, doi: 10.18632/oncotarget.16607.
Lerner, L., Tao, J., Liu, Q., Nicoletti, R., Feng, B., Krieger, B., Mazsa, E., Siddiquee, Z., Wang, R., Huang, L., Shen, L., Lin, J., Vigano, A., Chiu, M. I., Weng, Z., Winston, W., Weiler, S., and Gyuris, J. (2016) MAP3K11/GDF15 axis is a critical driver of cancer cachexia, J. Cachexia Sarcopenia Muscle, 7, 467-482, doi: 10.1002/jcsm.12077.
Jones, J. E., Cadena, S. M., Gong, C., Wang, X., Chen, Z., Wang, S. X., Vickers, C., Chen, H., Lach-Trifilieff, E., Hadcock, J. R., and Glass, D. J. (2018) Supraphysiologic administration of GDF11 induces Cachexia in part by upregulating GDF15, Cell Rep., 22, 1522-1530, doi: 10.1016/j.celrep.2018.01.044.
Bartke, A., and Darcy, J. (2017) GH and ageing: pitfalls and new insights, Best Pract. Res. Clin. Endocrinol. Metab., 31, 113-125, doi: 10.1016/j.beem.2017.02.005.
Rudman, D., Feller, A. G., Nagraj, H. S., Gergans, G. A., Lalitha, P. Y., Goldberg, A. F., Schlenker, R. A., Cohn, L., Rudman, I. W., and Mattson, D. E. (1990) Effects of human growth hormone in men over 60 years old, N. Engl. J. Med., 323, 1-6, doi: 10.1056/NEJM199007053230101.
Sattler, F. R. (2013) Growth hormone in the aging male, Best Pract. Res. Clin. Endocrinol. Metab., 27, 541-555, doi: 10.1016/j.beem.2013.05.003.
Kim, J., and Guan, K.-L. (2019) mTOR as a central hub of nutrient signalling and cell growth, Nat. Cell Biol., 21, 63-71, doi: 10.1038/s41556-018-0205-1.
Weihrauch, M., and Handschin, C. (2018) Pharmacological targeting of exercise adaptations in skeletal muscle: benefits and pitfalls, Biochem. Pharmacol., 147, 211-220, doi: 10.1016/j.bcp.2017.10.006.
Kaczka, P., Michalczyk, M. M., Jastrząb, R., Gawelczyk, M., and Kubicka, K. (2019) Mechanism of action and the effect of beta-hydroxy-beta-methylbutyrate (HMB) supplementation on different types of physical performance – a systematic review, J. Hum. Kinet., 68, 211-222, doi: 10.2478/hukin-2019-0070.
Cruz-Jentoft, A. J. (2018) Beta-hydroxy-beta-methyl butyrate (HMB): from experimental data to clinical evidence in sarcopenia, Curr. Protein Pept. Sci., 19, 668-672, doi: 10.2174/1389203718666170529105026.
Vyssokikh, M. Y., Holtze, S., Averina, O. A., Lyamzaev, K. G., Panteleeva, A. A., Marey, M. V., Zinovkin, R. A., Severin, F. F., Skulachev, M. V., Fasel, N., Hildebrandt, T. B., and Skulachev, V. P. (2020) Mild depolarization of the inner mitochondrial membrane is a crucial component of an anti-aging program, Proc. Natl. Acad. Sci. USA, 117, 6491-6501, doi: 10.1073/pnas.1916414117.
Sokolov, S. S., Markova, O. V., Nikolaeva, K. D., Fedorov, I. A., and Severin, F. F. (2017) Triosephosphates as intermediates of the Crabtree effect, Biochemistry (Moscow), 82, 458-464, doi: 10.1134/S0006297917040071.
Chen, C., Zhou, M., Ge, Y., and Wang, X. (2020) SIRT1 and aging related signaling pathways, Mech. Ageing Dev., 187, 111215, doi: 10.1016/j.mad.2020.111215.
Santos, L., Escande, C., and Denicola, A. (2016) Potential modulation of sirtuins by oxidative stress, Oxid. Med. Cell. Longev., 2016, 9831825, doi: 10.1155/2016/9831825.
Yamaza, H., Komatsu, T., Wakita, S., Kijogi, C., Park, S., Hayashi, H., Chiba, T., Mori, R., Furuyama, T., Mori, N., and Shimokawa, I. (2010) FoxO1 is involved in the antineoplastic effect of calorie restriction, Aging Cell, 9, 372-382, doi: 10.1111/j.1474-9726.2010.00563.x.
Badreh, F., Joukar, S., Badavi, M., Rashno, M., and Dehesh, T. (2019) The effects of age and fasting models on blood pressure, insulin/glucose profile, and expression of longevity proteins in male rats, Rejuvenation Res., doi: 10.1089/rej.2019.2205.
Rodríguez-Prados, J.-C., Través, P. G., Cuenca, J., Rico, D., Aragonés, J., Martín-Sanz, P., Cascante, M., and Boscá, L. (2010) Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation, J. Immunol., 185, 605-614, doi: 10.4049/jimmunol.0901698.
Mann, G. E., Yudilevich, D. L., and Sobrevia, L. (2003) Regulation of amino acid and glucose transporters in endothelial and smooth muscle cells, Physiol. Rev., 83, 183-252, doi: 10.1152/physrev.00022.2002.
Karavaeva, I. E., Golyshev, S. A., Smirnova, E. A., Sokolov, S. S., Severin, F. F., and Knorre, D. A. (2017) Mitochondrial depolarization in yeast zygotes inhibits clonal expansion of selfish mtDNA, J. Cell Sci., 130, 1274-1284, doi: 10.1242/jcs.197269.
Caldeira da Silva, C. C., Cerqueira, F. M., Barbosa, L. F., Medeiros, M. H. G., and Kowaltowski, A. J. (2008) Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity, Aging Cell, 7, 552-560, doi: 10.1111/j.1474-9726.2008.00407.x.
Pelletier, A., and Coderre, L. (2007) Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes, Am. J. Physiol. Endocrinol. Metab., 292, E1325-E1332, doi: 10.1152/ajpendo.00186.2006.
Zakharova, V. V., Pletjushkina, O. Y., Galkin, I. I., Zinovkin, R. A., Chernyak, B. V., Krysko, D. V., Bachert, C., Krysko, O., Skulachev, V. P., and Popova, E. N. (2017) Low concentration of uncouplers of oxidative phosphorylation decreases the TNF-induced endothelial permeability and lethality in mice, Biochim. Biophys. Acta Mol. Basis Dis., 1863, 968-977, doi: 10.1016/j.bbadis.2017.01.024.
Lee, Y., Heo, G., Lee, K. M., Kim, A. H., Chung, K. W., Im, E., Chung, H. Y., and Lee, J. (2017) Neuroprotective effects of 2,4-dinitrophenol in an acute model of Parkinson’s disease, Brain Res., 1663, 184-193, doi: 10.1016/j.brainres.2017.03.018.
Kishimoto, Y., Johnson, J., Fang, W., Halpern, J., Marosi, K., Liu, D., Geisler, J. G., and Mattson, M. P. (2020) A mitochondrial uncoupler prodrug protects dopaminergic neurons and improves functional outcome in a mouse model of Parkinson’s disease, Neurobiol. Aging, 85, 123-130, doi: 10.1016/j.neurobiolaging.2019.09.011.
Coll, A. P., Chen, M., Taskar, P., Rimmington, D., Patel, S., et al. (2020) GDF15 mediates the effects of metformin on body weight and energy balance, Nature, 578, 444-448, doi: 10.1038/s41586-019-1911-y.
Klaus, S., and Ost, M. (2020) Mitochondrial uncoupling and longevity – a role for mitokines? Exp. Gerontol., 130, 110796, doi: 10.1016/j.exger.2019.110796.
Spiering, M. J. (2019) The mystery of metformin, J. Biol. Chem., 294, 6689-6691, doi: 10.1074/jbc.CL119.008628.
Cadenas, E., Boveris, A., Ragan, C. I., and Stoppani, A. O. (1977) Production of superoxide radicals and hydrogen peroxide by NADH-ubiquinone reductase and ubiquinol-cytochrome c reductase from beef-heart mitochondria, Arch. Biochem. Biophys., 180, 248-257, doi: 10.1016/0003-9861(77)90035-2.
Mirphy, M. P. (2009) How mitochondria produce reactive oxygen species, Biochem. J., 417, 1-13, doi: 10.1042/BJ20081386.
Lenaz, G., Tioli, G., Falasca, A. I., and Genova, M. L. (2016) Complex I function in mitochondrial supercomplexes, Biochim. Biophys. Acta, 1857, 991-1000, doi: 10.1016/j.bbabio.2016.01.013.
Hunt, P. R., Son, T. G., Wilson, M. A., Yu, Q.-S., Wood, W. H., Zhang, Y., Becker, K. G., Greig, N. H., Mattson, M. P., Camandola, S., and Wolkow, C. A. (2011) Extension of lifespan in C. elegans by naphthoquinones that act through stress hormesis mechanisms, PLoS One, 6, e21922, doi: 10.1371/journal.pone.0021922.
Sakurai, H., and Ota, A. (2011) Regulation of chaperone gene expression by heat shock transcription factor in Saccharomyces cerevisiae: importance in normal cell growth, stress resistance, and longevity, FEBS Lett., 585, 2744-2748, doi: 10.1016/j.febslet.2011.07.041.
Badave, K. D., Khan, A. A., and Rane, S. Y. (2016) Anticancer vitamin K3 analogs: a review, Anticancer Agents Med. Chem., 16, 1017-1030, doi: 10.2174/1871520616666160310143316.
Sies, H., and Jones, D. P. (2020) Reactive oxygen species (ROS) as pleiotropic physiological signalling agents, Nat. Rev. Mol. Cell Biol., doi: 10.1038/s41580-020-0230-3.
Wiel, C., Le Gal, K., Ibrahim, M. X., Jahangir, C. A., Kashif, M., Yao, H., Ziegler, D. V., Xu, X., Ghosh, T., Mondal, T., Kanduri, C., Lindahl, P., Sayin, V. I., and Bergo, M. O. (2019) BACH1 stabilization by antioxidants stimulates lung cancer metastasis, Cell, 178, 330-345, doi: 10.1016/j.cell.2019.06.005.
Skulachev, M. V., and Skulachev, V. P. (2017) Programmed aging of mammals: proof of concept and prospects of biochemical approaches for anti-aging therapy, Biochemistry (Moscow), 82, 1403-1422, doi: 10.1134/S000629791712001X.
Isaev, N. K., Stelmashook, E. V., Genrikhs, E. E., Korshunova, G. A., Sumbatyan, N. V., Kapkaeva, M. R., and Skulachev, V. P. (2016) Neuroprotective properties of mitochondria-targeted antioxidants of the SkQ-type, Rev. Neurosci., 27, 849-855, doi: 10.1515/revneuro-2016-0036.
Baksheeva, V. E., Gancharova, O. S., Tiulina, V. V., Iomdina, E. N., Zamyatnin, A. A. Jr., Philippov, P. P., Zernii, E. Y., and Senin, I. I. (2018) Iatrogenic damage of eye tissues: current problems and possible solutions, Biochemistry (Moscow), 83, 1563-1574, doi: 10.1134/S0006297918120143.
Sies, H., Berndt, C., and Jones, D. P. (2017) Oxidative stress, Annu. Rev. Biochem., 86, 715-748, doi: 10.1146/annurev-biochem-061516-045037.
Marinho, H. S., Real, C., Cyrne, L., Soares, H., and Antunes, F. (2014) Hydrogen peroxide sensing, signaling and regulation of transcription factors, Redox Biol., 2, 535-562, doi: 10.1016/j. redox.2014.02.006.
Borisova-Mubarakshina, M. M., Vetoshkina, D. V., and Ivanov, B. N. (2019) Antioxidant and signaling functions of the plastoquinone pool in higher plants, Physiol. Plant., 166, 181-198, doi: 10.1111/ppl.12936.
Borisova-Mubarakshina, M. M., Naydov, I. A., and Ivanov, B. N. (2018) Oxidation of the plastoquinone pool in chloroplast thylakoid membranes by superoxide anion radicals, FEBS Lett., 592, 3221-3228, doi: 10.1002/1873-3468.13237.
Severin, F. F., and Skulachev, V. P. (2009) Programmed cell death as a target to interrupt the aging program, Adv. Gerontol., 22, 37-48.
NCD Countdown 2030 collaborators (2018) NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4, Lancet, 392, 1072-1088, doi: 10.1016/S0140-6736(18)31992-5.
Gong, J. B., Yu, X. W., Yi, X. R., Wang, C. H., and Tuo, X. P. (2018) Epidemiology of chronic noncommunicable diseases and evaluation of life quality in elderly, Aging Med., 1, 64-66, doi: 10.1002/agm2.12009.
Funding
This work was supported by the Russian Foundation for Basic Research (project No. 19-14-50642).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain description of studies involving humans or animals as research subjects performed by any of the authors. The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Sokolov, S., Severin, F. Manipulating Cellular Energetics to Slow Aging of Tissues and Organs. Biochemistry Moscow 85, 651–659 (2020). https://doi.org/10.1134/S0006297920060024
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006297920060024