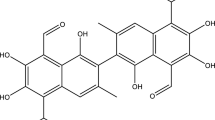
Abstract
Herein environment-friendly solvent 2-propanol-water (95:5 v/v) acidified with 0.5 M oxalic acid was utilized to extract gossypol, a naturally occurring pigment of cottonseed. This polyphenolic pigment gossypol acts as a toxin to cottonseed oil and protein during processing of cottonseed by imparting a dark colouration. The parameters affecting the solvent extraction process, such as temperature, solvent-to-seed ratio, acid concentration and contact time, were studied and optimized along with evaluation of kinetic and thermodynamic parameters. The gossypol extraction followed pseudo-second-order kinetics and the kinetics mechanism displayed the diffusion of gossypol from solid phase to the liquid phase. The extraction process was internal diffusion controlled, and internal solid diffusion was the rate controlling step. The optimum conditions for extraction of gossypol were obtained experimentally as 0.5 M oxalic acid concentration, temperature of 348 K, solvent-to-seed ratio of 15 and an extraction time of 180 min, exhibiting maximum gossypol extraction of 95.43% under these conditions. The activation energy of extraction was found out to be 6.099 kJ/mol while the gossypol extraction process was found out to be endothermic in nature, viable and increased the degree of randomness of extraction process. 2-Propanol-water solvent proved to be a potent green solvent for extraction of gossypol from cottonseed.








Similar content being viewed by others
References
Surinder Singh, S.K.; Kansal, Sharma S.K.: Extraction of gossypol from cottonseed. Rev. Adv. Sci. Eng. 4, 301–318 (2015). https://doi.org/10.1166/rase.2015.1105
Price, W.D.; Lovell, R.A.; McChesney, D.G.: Naturally occurring toxins in feedstuffs: center for veterinary medicine perspective. J. Anim. Sci. 71(9), 2556–2562 (1993)
Coutinho, E.M.: Gossypol: a contraceptive for men. Contraception 65, 259–263 (2002)
Pelitire, S.M.; Dowd, M.K.; Cheng, H.N.: Acidic solvent extraction of gossypol from cottonseed meal. Anim. Feed Sci. Technol. 195, 120–128 (2014). https://doi.org/10.1016/j.anifeedsci.2014.06.005
Berardi, L.C.; Frampton, V.L.: Note on gossypol and its relation to color fixation in cottonseed oil. J. Am. Oil Chem. Soc. 34(8), 399–401 (1957). https://doi.org/10.1007/BF02637891
Aguieiras, E.C.G.; De Barros, D.S.N.; Fernandez-lafuente, R.; Freire, D.M.G.: Production of lipases in cottonseed meal and application of the fermented solid as biocatalyst in esterification and transesterification reactions. Renew. Energy. 130, 574–581 (2019). https://doi.org/10.1016/j.renene.2018.06.095
Wedegaertner, T.; Rathore, K.: Elimination of gossypol in cottonseed will improve its utilization. Procedia Environ. Sci. 29, 124–125 (2015). https://doi.org/10.1016/j.proenv.2015.07.212
Vadehra, D.V.; Kalla, N.R.; Saxena, M.; et al.: Antimicrobial activity of gossypol acetic acid. IRCS Med. Sci. 13, 10–11 (1985)
Wang, X.; Howell, C.P.; Chen, F.; Yin, J.; Jiang, Y.: Gossypol-a polyphenolic compound from cotton plant. Adv. Food Nutr. Res. 58, 215–263 (2009). https://doi.org/10.1016/S1043-4526(09)58006-0
Lan, L.; Appelman, C.; Smith, A.R.; Yu, J.; Larsen, S.; Marquez, R.T.; Liu, H.; Wu, X.; Gao, P.; Roy, A.; Anbanandam, A.; Gowthaman, R.; Karanicolas, J.; De Guzman, R.N.; Rogers, S.; Aubé, J.; Ji, M.; Cohen, R.S.; Neufeld, K.L.; Xu, L.: Natural product (-)-gossypol inhibits colon cancer cell growth by targeting RNA-binding protein Musashi-1. Mol. Oncol. 9, 1406–1420 (2015). https://doi.org/10.1016/j.molonc.2015.03.014
Zeng, Y.; Ma, J.; Xu, L.; Wu, D.: Natural product gossypol and its derivatives in precision cancer medicine. Curr. Med. Chem. (2017). https://doi.org/10.2174/0929867324666170523123655. (E pub ahead of print)
Tian, X.; Ruan, J.; Huang, J.; Fang, X.; Mao, Y.; Wang, L.; Chen, X.; Yang, C.: Gossypol: phytoalexin of cotton. Sci China Life Sci 59, 122–129 (2016). https://doi.org/10.1007/s11427-016-5003-z
Lyman, C.M.; Baliga, B.P.; Slay, M.W.: Reactions of proteins with gossypol. Arch. Biochem. Biophys. 84(2), 486–497 (1959). https://doi.org/10.1016/0003-9861(59)90610-1
Eagle, E.; Hall, C.M.; Castillon, L.E.; Miller, C.B.: Effect of fractionation and treatment on the acute oral toxicity and on the gossypol and gossypurpurin content of cottonseed pigment glands. J. Am. Oil Chem. Soc. 27(8), 301–303 (1950). https://doi.org/10.1007/BF02649313
Batson, D.M.; Thurbur, F.H.; Altschul, A.M.: The effect of screw-press and hydraulic-press processing conditions on pigment glands in cottonseed. J. Am. Oil Chem. Soc. 28(11), 468–472 (1951). https://doi.org/10.1007/BF02613062
Gribbins, G.H.: The reduction of free gossypol in cottonseed by pressure cooking. J. Am. Oil Chem. Soc. 28(2), 41–45 (1951). https://doi.org/10.1007/BF02612086
Thurber, F.H.; Vix, H.L.E.; Pons Jr., W.A.; Crovetto, A.J.; Knoepfler, N.B.: The effect of processing conditions on the properties of screw-press cottonseed meal and oil. J. Am. Oil Chem. Soc. 31(9), 384–388 (1954). https://doi.org/10.1007/BF02545516
Smith, A.K.: Practical considerations in commercial utilization of oil seeds. J. Am. Oil Chem. Soc. 48(1), 38–42 (1971). https://doi.org/10.1007/BF02673240
Vix, H.L.E.; Eaves, P.H.; Gardner Jr., H.K.; Lambou, M.G.: Degossypolised cottonseed flour-the liquid cyclone process. J. Am. Oil Chem. Soc. 48(10), 611–615 (1971). https://doi.org/10.1007/BF02544573
Hron, R.J.; Abraham, G.; Kuk, M.S.; Fisher, G.S.: Acidic ethanol extraction of cottonseed. J. Am. Oil Chem. Soc. 69(9), 951–952 (1992). https://doi.org/10.1007/BF02636351
Ho, Y.; Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C.: Kinetics and model building of leaching of water-soluble compounds of Tilia sapwood. Sep. Purif. Technol. 45(3), 169–173 (2005). https://doi.org/10.1016/j.seppur.2005.03.007
Saxena, D.K.; Sharma, S.K.; Sambi, S.S.: Kinetics and thermodynamics of gossypol extraction from defatted cottonseed meal by ethanol. Polish J. Chem. Technol. 14(2), 29–34 (2012). https://doi.org/10.2478/v10026-012-0067-4
Saxena, D.K.; Sharma, S.K.; Sambi, S.S.: Kinetics and thermodynamics of gossypol extraction from defatted cottonseed meal by ethanol Acidified by Oxalic acid. Int. J. Sci. Res. 4(8), 1967–1971 (2015)
Li, Z.; Smith, K.H.; Stevens, G.W.: The use of environmentally sustainable bio-derived solvents in solvent extraction applications—a review. Chinese J. Chem. Eng. 24(2), 215–220 (2016). https://doi.org/10.1016/j.cjche.2015.07.021
Kenar, J.A.: Reaction chemistry of gossypol and its derivatives. J. Am. Oil Chem. Soc. 83(4), 269–302 (2006). https://doi.org/10.1007/s11746-006-1203-1
Vander Jagt, D.L.; Deck, L.M.; Royer, R.E.: Gossypol prototype of inhibitors targeted to dinucleotide folds. Curr. Med. Chem. 7(4), 479–498 (2000). https://doi.org/10.2174/0929867003375119
Arnold, L.K.; Juhl, W.G.: The reduction of free gossypol in cottonseed flakes during solvent extraction. J. Am. Oil Chem. Soc. 32(3), 151–152 (1955). https://doi.org/10.1007/BF02640325
Bressani, R.; Jarquın, R.; Elias, L.G.: Cottonseed flour, free and total gossypol, epsilon-amino lysine and biological evaluation of cottonseed meals and flours in Central America. J. Agric. Food Chem. 12(3), 278–282 (1964). https://doi.org/10.1021/jf60133a027
Fernandez, S.R.; Zhang, Y.; Parsons, C.M.: Dietary formulation with cottonseed meal on a total amino acid versus a digestible amino acid basis. Poult. Sci. 74(7), 1168–1179 (1995). https://doi.org/10.3382/ps.0741168
Dowd, M.K.; Pelitire, S.M.: Isolation of 6-methoxy gossypol and 6, 6’-dimethoxy gossypol from Gossypium barbadense Sea Island cotton. J. Agric. Food Chem. 54, 3265–3270 (2006)
Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Robert McElroy, C.; Sherwood, J.: Tools and techniques for solvent selection: green solvent selection guides. Sustain. Chem. Process. 4(7), 1–24 (2016). https://doi.org/10.1186/s40508-016-0051-z
Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; Mcelroy, C.R.; Abou-shehada, S.; Dunn, P.J.: CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 18, 288–296 (2016). https://doi.org/10.1039/c5gc01008j
BIS: Specification for edible cottonseed flour (solvent extrcated) IS:4876.1986 Bureau of Indian Standards. BIS:1–16 (1986) https://ia800409.us.archive.org/8/items/gov.in.is.4876.1986/is.4876.1986.pdf. Accessed on 24 Jan 2020.
Singh, S.; Sharma, S.K.; Kansal, S.K.: Batch extraction of gossypol from cottonseed meal using mixed solvent system and its kinetic modeling. Chem. Eng. Commun. 206(12), 1608–1617 (2019). https://doi.org/10.1080/00986445.2018.1558214
Harouna-Oumarou, H.A.; Fauduet, H.; Porte, C.; Ho, Y.S.: Comparison of kinetic models for the aqueous solid-liquid extraction of Tilia sapwood a continuous stirred tank reactor. Chem. Eng. Commun. 194(4), 537–552 (2007). https://doi.org/10.1080/00986440600992511
Ho, Y.S.; McKay, G.: Pseudo-second order model for sorption processes. Process Biochem. 34(5), 451–465 (1999). https://doi.org/10.1016/S0032-9592(98)00112-5
Pohndorf, R.S.; Jr, T.R.S.C.; Pinto, L.A.A.: Kinetics and thermodynamics adsorption of carotenoids and chlorophylls in rice bran oil bleaching. J. Food Eng. 185, 9–16 (2016). https://doi.org/10.1016/j.jfoodeng.2016.03.028
Kumar, K.V.; Khaddour, I.A.; Gupta, V.K.: A pseudo second-order kinetic expression for dissolution kinetic profiles of solids in solutions. Ind. Eng. Chem. Res. 49, 7257–7262 (2010). https://doi.org/10.1021/ie1010228
Sant, V.; Brandelli, A.; Damasceno, L.; Marczak, F.; Cristina, I.: Kinetic modeling of total polyphenol extraction from grape marc and characterization of the extracts 100, 82–87 (2012). https://doi.org/10.1016/j.seppur.2012.09.004
Dutta, R.; Sarkar, U.; Mukherjee, A.: Pseudo-kinetics of batch extraction of Crotalaria juncea (Sunn hemp) seed oil using 2-propanol. Ind. Crop. Prod. 87, 9–13 (2016). https://doi.org/10.1016/j.indcrop.2016.04.006
Rashid, T.; Gnanasundaram, N.; Appusamy, A.; Fai, C.: Industrial crops & products enhanced lignin extraction from different species of oil palm biomass: kinetics and optimization of extraction conditions. Ind. Crop. Prod. 116, 122–136 (2018). https://doi.org/10.1016/j.indcrop.2018.02.056
Sirry, S.M.; Aldakhil, F.; Alharbi, O.M.L.; Ali, I.: Chemically treated date stones for uranium (VI) uptake and extraction in aqueous solutions. J. Mol. Liq. 273, 192–202 (2019). https://doi.org/10.1016/j.molliq.2018.10.018
Weber, W.J.; Morris, J.C.: Kinetics of adsorption on carbon from solution. J. Sanitary Eng. Div. 89(2), 31–60 (1963)
Yoro, K.O.; Amosa, M.K.; Sekoai, P.T.; Mulopo, J.; Daramola, M.O.: Diffusion mechanism and effect of mass transfer limitation during the adsorption of CO2 by polyaspartamide in a packed-bed unit. Int. J. Sustain. Eng. (2019). https://doi.org/10.1080/19397038.2019.1592261
Hron Sr., R.J.; Kuk, M.S.; Abraham, G.; Wan, P.J.: Ethanol extraction of oil, gossypol and aflatoxin from cottonseed. J. Am. Oil Chem. Soc. 71(4), 417–421 (1994). https://doi.org/10.1007/BF02540523
Chi, R.; Tian, J.; Gao, H.; Zhou, F.; Liu, M.; Wang, C.; Wu, Y.: Kinetics of leaching flavonoids from pueraria lobata with ethanol. Chinese J. Chem. Eng. 14(3), 402–406 (2006). https://doi.org/10.1016/S1004-9541(06)60091-8
Rakotondramasy-Rabesiaka, L.; Havet, J.L.; Porte, C.; Fauduet, H.: Solid-liquid extraction of protopine from Fumaria officinalis L-analysis determination, kinetic reaction and model building. Sep. Purif. Technol. 54(2), 253–261 (2007). https://doi.org/10.1016/j.seppur.2006.09.015
Miyake, Y.; Ishida, H.; Tanaka, S.; Kolev, S.D.: Theoretical analysis of the pseudo-second order kinetic model of adsorption. Application to the adsorption of Ag (I) to mesoporous silica microspheres functionalized with thiol groups. Chem. Eng. J. 218, 350–357 (2013). https://doi.org/10.1016/j.cej.2012.11.089
Simeonov, E.; Tsibranska, I.; Minchev, A.: Solid-liquid extraction from plants-experimental kinetics and modelling. Chem. Eng. J. 73(3), 255–259 (1999). https://doi.org/10.1016/S1385-8947(99)00030-3
Wongkittipong, R.; Prat, L.; Damronglerd, S.; Gourdon, C.: Solid-liquid extraction of andrographolide from plants-experimental study, kinetic reaction and model. Sep. Purif. Technol. 40(2), 147–154 (2004). https://doi.org/10.1016/j.seppur.2004.02.002
Ho, Y.S.: Removal of copper ions from aqueous solution by tree fern. Water Res. 37, 2323–2330 (2003)
Bispo, S.; Martins, M.A.; Caneschi, A.L.; Rafael, P.; Aguilar, M.; Selia, J.: Kinetics and thermodynamics of oil extraction from Jatropha curcas L using ethanol as a solvent. J. Chem. Eng. Int. (2015). https://doi.org/10.1155/2015/871236
Meziane, S.; Kadi, H.: Kinetics and thermodynamics of oil extraction from olive cake. J. Am. Oil Chem. Soc. 85, 391–396 (2008)
Kostic, M.D.; Jokovic, N.M.; Stamenkovic, O.S.; Rajkovic, K.M.; Milic, P.S.; Veljkovic, V.B.: The kinetics and thermodynamics of hempseed oil extraction by n-hexane. Ind. Crop. Prod. 52, 679–686 (2014)
Johnson, L.A.; Lusas, E.W.: Comparison of alternative solvents for oils extraction. J. Am. Oil Chem. Soc. 60(2), 229–241 (1983)
Stamenković, O.S.; Kostić, M.D.; Tasić, M.B.; Djalović, I.G.; Mitrović, P.M.; Biberdžić, M.O.; Veljković, V.B.: Kinetic, thermodynamic and optimization study of the corn germ oil extraction process. Food Bioprod. Process. 120, 91–103 (2020). https://doi.org/10.1016/j.fbp.2019.12.013
Stamenković, O.S.; Djalović, I.G.; Kostić, M.D.; Mitrović, P.M.; Veljković, V.B.: Optimization and kinetic modeling of oil extraction from white mustard (Sinapis alba L.) seeds. Ind. Crops Prod. 121, 132–141 (2018). https://doi.org/10.1016/j.indcrop.2018.05.001
Agu, C.M.; Kadurumba, C.H.; Agulanna, A.C.; Aneke, O.O.; Agu, I.E.; Eneh, J.N.: Nonlinear kinetics, thermodynamics, and parametric studies of colocynthis vulgaris shrad seeds oil extraction. Ind. Crops Prod. 123, 386–400 (2018). https://doi.org/10.1016/j.indcrop.2018.06.074
Milić, P.S.; Rajković, K.M.; Bekrić, D.M.; Stamenković, O.S.; Veljković, V.B.: The kinetic and thermodynamic analysis of ultrasound-extraction of minerals from aerial parts of white lady’s bedstraw (Galium mollugo L). Chem. Eng. Res. Des. 92, 1399–1409 (2014). https://doi.org/10.1016/j.cherd.2013.10.024
Dagostin, J.L.A.; Carpiné, D.; Corazza, M.L.: Extraction of soybean oil using ethanol and mixtures with alkyl esters (biodiesel) as co-solvent: kinetics and thermodynamics. Ind. Crops Prod. 74, 69–75 (2015). https://doi.org/10.1016/j.indcrop.2015.04.054
Amarante, R.C.A.; Oliveira, P.M.; Schwantes, F.K.; Morón-Villarreyes, J.A.: Oil extraction from castor cake using ethanol: kinetics and thermodynamics. Ind. Eng. Chem. Res. 53, 6824–6829 (2014). https://doi.org/10.1021/ie500508n
Liadakis, G.N.; Floridis, A.; Tzia, C.; Oreopoulou, V.: Protein isolates with reduced gossypol content from screw-pressed cottonseed meal. J. Agric. Food Chem. 41(6), 918–922 (1993). https://doi.org/10.1021/jf00030a016
Kuk, M.S.; Hron Sr., R.J.: Cottonseed extraction with new solvent system: isohexane and alcohol. J. Am. Oil Chem. Soc. 75(8), 927–930 (1998). https://doi.org/10.1007/s11746-998-0268-4
Kuk, M.S.; Tetlow, R.; Dowd, M.K.: Cotton seed extraction with mixture of acetone and hexane. J. Am. Oil Chem. Soc. 82(8), 609–612 (2005). https://doi.org/10.1007/s11746-005-1117-y
Acknowledgements
The authors are highly grateful to Guru Gobind Singh Indraprastha University, Dwarka Sector-16, Delhi, and Panjab University, Chandigarh, for providing necessary facilities and technical support. This work was supported by TEQIP-III grant (MHRD, Govt. of India; 2017-2020) of Dr. S. S. Bhatnagar University Institute of Chemical Engineering and Technology, Panjab University, Chandigarh.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors hereby state that they have no conflicts of interest due to present report.
Rights and permissions
About this article
Cite this article
Singh, S., Sharma, S.K. & Kansal, S.K. Extraction of Natural Pigment Gossypol from Defatted Cottonseed Using 2-Propanol-Water Green Solvent, Its Kinetics and Thermodynamic Study. Arab J Sci Eng 45, 7539–7550 (2020). https://doi.org/10.1007/s13369-020-04665-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-020-04665-6