Abstract
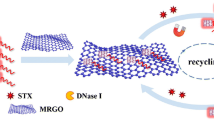
Cylindrospermopsin (CYN) is one of the most concerning cyanotoxins due to its potential toxicity and spreading to various environments including drinking water. CYN has potential interferences with human and animal metabolic pathways, which influence the functions of organs including liver, kidneys, lungs, etc. CYN is involved in the inhibition of protein synthesis and detachment of ribosomes from the endoplasmic reticulum membrane. It also interacts with soluble proteins, which are associated with protein translations. It is believed that cytochrome 450 is responsible for the rapid toxicity of CYN. Researchers are urged to develop a high-throughput screening method for the detection of CYN in water. Construction of low cost, rapid, and sensitive analytical methods for the detection of CYN is challenging. Here, we used graphene oxide (GO) as the fluorescence sensing platform for probing the high affinity of the short aptamer derived from the wild-type long aptamer-CYN sensing. The biosensor construction involved two steps: first, quenching the fluorescence of fluorescent-labelled truncated aptamer using GO as a quencher and, second, fluorescence recovery in the presence of CYN by competitive binding between the target and GO. One of the truncate aptamers has a 12-fold higher affinity and enhances sensitivity compared to the long aptamer sequence. The limit of detection of the high affinity truncated aptamer is 17 pM which is 6-fold lower than the long aptamer (100 pM). The sensor specifically detects CYN in the presence of other potential interfering toxins. The performance of the sensor was validated using CYN spiked tap water with very good recovery percentage. A rapid and highly sensitive detection of CYN from water resources has been achieved using this method.








Similar content being viewed by others
References
Moreira C, Azevedo J, Antunes A, Vasconcelos V. Cylindrospermopsin: occurrence, methods of detection and toxicology. J Appl Microbiol. 2013;114:605–20.
Armah A, Hiskia A, Kaloudis T, Chernoff N, Hill D, Antoniou MG, et al. A review on cylindrospermopsin: the global occurrence, detection, toxicity and degradation of a potent cyanotoxin. Environ Sci Process Impacts. 2013;15:1979–2003.
Chiswell RK, Shaw GR, Eaglesham G, Smith MJ, Norris RL, Seawright AA, et al. Stability of cylindrospermopsin, the toxin from the cyanobacterium, cylindrospermopsis raciborskii: effect of pH, temperature, and sunlight on decomposition. Environ Toxicol. 1999;14:155–61.
Wörmer L, Huerta-Fontela M, Cirés S, Carrasco D, Quesada A. Natural photodegradation of the cyanobacterial toxins microcystin and cylindrospermopsin. Environ Sci Technol. 2010;44:3002–7.
Farrer D, Counter M, Hillwig R, Cude C. Health-based cyanotoxin guideline values allow for cyanotoxin-based monitoring and efficient public health response to cyanobacterial blooms. Toxins. 2015;7:457–77.
Ohtani I, Moore RE, Runnegar MTC. Cylindrospermopsin: a potent hepatotoxin from the blue-green alga Cylindrospermopsis raciborskii. J Am ChemSoc. 1992;114:7941–2.
Hiskia A, Spoof L, Kaloudis T, Meriluoto J. Determination of cyanotoxins by high-performance liquid chromatography with photodiode array. Handbook of cyanobacterial monitoring and cyanotoxin analysis, 2017, 205.
Welker M, Bickel H, Fastner J. HPLC-PDA detection of cylindrospermopsin—opportunities and limits. Water Res. 2002;36:4659–63.
Gallo P, Fabbrocino S, Cerulo MG, Ferranti P, Bruno M, Serpe L. Determination of cylindrospermopsin in freshwaters and fish tissue by liquid chromatography coupled to electrospray ion trap mass spectrometry. Rapid Commun Mass Sp. 2009;23:3279–84.
Blahova L, Oravec M, Maršálek B, Šejnohová L, Šimek Z, Bláha L. The first occurrence of the cyanobacterial alkaloid toxin cylindrospermopsin in the Czech Republic as determined by immunochemical and LC/MS methods. Toxicon. 2009;53:519–24.
Guzmán-Guillén R, Prieto AI, Gonzalez AG, Soria-Díaz ME, Cameán AM. Cylindrospermopsin determination in water by LC-MS/MS: optimization and validation of the method and application to real samples. Environ Toxicol Chem. 2012;31:2233–8.
Esterhuizen-Londt M, Kühn S, Pflugmacher S. Development and validation of an in-house quantitative analysis method for cylindrospermopsin using hydrophilic interaction liquid chromatography–tandem mass spectrometry: quantification demonstrated in 4 aquatic organisms. Environ Toxicol Chem. 2015;34:2878–83.
Yen H-K, Lin T-F, Liao P-C. Simultaneous detection of nine cyanotoxins in drinking water using dual solid-phase extraction and liquid chromatography–mass spectrometry. Toxicon. 2011;58:209–18.
Wei D. Writable electrochemical energy source based on graphene oxide. Sci Rep. 2015;5:15173.
Chidembo A, Aboutalebi SH, Konstantinov K, Salari M, Winton B, Yamini SA, et al. Globular reduced graphene oxide-metal oxide structures for energy storage applications. Energy Environ Sci. 2012;5:5236–40.
Dideikin AT, Vul AY. Graphene oxide and derivatives: the place in graphene family. Front Phys. 2019;6:149.
Shareena TPD, McShan D, Dasmahapatra AK, Tchounwou PB. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano-Micro Let. 2018;10:53.
Chung C, Kim Y-K, Shin D, Ryoo S-R, Hong BH, Min D-H. Biomedical applications of graphene and graphene oxide. Acc Chem Res. 2013;46:2211–24.
Peña-Bahamonde J, Nguyen HN, Fanourakis SK, Rodrigues DF. Recent advances in graphene-based biosensor technology with applications in life sciences. J Nanobiotechnol. 2018;16:75.
Lu Z, Chen X, Wang Y, Zheng X, Li CM. Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta. 2015;182:571–8.
Qu H, Csordas AT, Wang J, Oh SS, Eisenstein MS, Soh HT. Rapid and label-free strategy to isolate aptamers for metal ions. ACS Nano. 2016;10:7558–65.
Eissa S, Siddiqua A, Chinnappan R, Zourob MM. Electrochemical SELEX technique for the selection of DNA aptamers against the small molecule 11-deoxycortisol. ACS Appl Bio Mater. 2019.
Chinnappan R, Eissa S, Alotaibi A, Siddiqua A, Alsager OA, Zourob M. In vitro selection of DNA aptamers and their integration in a competitive voltammetric biosensor for azlocillin determination in waste water. Anal Chim Acta. 2020;1101:149–56.
Aljohani MM, Chinnappan R, Eissa S, Alsager OA, Weber K, Cialla-May D, et al. In vitro selection of specific DNA Aptamers against the anti-coagulant dabigatran etexilate. Sci Rep. 2018;8:13290.
Chinnappan R, Rahamn AA, AlZabn R, Kamath S, Lopata AL, Abu-Salah KM, et al. Aptameric biosensor for the sensitive detection of major shrimp allergen, tropomyosin. Food Chem. 2019;126133.
Chinnappan R, AlAmer S, Eissa S, Rahamn AA, Abu Salah KM, Zourob M. Fluorometric graphene oxide-based detection of Salmonella enteritis using a truncated DNA aptamer. Microchim Acta. 2017;185:61.
Tang Z, Parekh P, Turner P, Moyer RW, Tan W. Generating aptamers for recognition of virus-infected cells. Clin Chem. 2009;55:813–22.
Chinnappan R, AlZabn R, Abu-Salah KM, Zourob M. An aptamer based fluorometric microcystin-LR assay using DNA strand-based competitive displacement. Microchim Acta. 2019;186:435.
Chinnappan R, AlZabn R, Mir TA, Bader M, Zourob M. Fluorometric determination of okadaic acid using a truncated aptamer. Microchim Acta. 2019;186:406.
Alhadrami HA, Chinnappan R, Eissa S, Rahamn AA, Zourob M. High affinity truncated DNA aptamers for the development of fluorescence based progesterone biosensors. Anal Biochem. 2017;525:78–84.
Elshafey R, Siaj M, Zourob M. In vitro selection, characterization, and biosensing application of high-affinity cylindrospermopsin-targeting aptamers. Anal Chem. 2014;86:9196–203.
Vorobyeva MA, Davydova AS, Vorobjev PE, Pyshnyi DV, Venyaminova AG. Key aspects of nucleic acid library design for in vitro selection. Int J Mol Sci. 2018;19:470.
Komarova N, Kuznetsov A. Inside the black box: what makes SELEX better? Molecules. 2019;24:3598.
Ku T-H, Zhang T, Luo H, Yen TM, Chen P-W, Han Y, et al. Nucleic acid aptamers: an emerging tool for biotechnology and biomedical sensing. Sensors. 2015;15:16281–313.
Adachi T, Nakamura Y. Aptamers: a review of their chemical properties and modifications for therapeutic application. Molecules. 2019;24:4229.
Zhao Z, Chen H, Ma L, Liu D, Wang Z. A label-free electrochemical impedance aptasensor for cylindrospermopsin detection based on thionine-graphene nanocomposites. Analyst. 2015;140:5570–7.
Lei L, Peng L, Yang Y, Han B-p. Development of time-resolved fluoroimmunoassay for detection of cylindrospermopsin using its novel monoclonal antibodies. Toxins. 2018;10:255.
Díez-Quijada L, Guzmán-Guillén R, Prieto Ortega AI, Llana-Ruíz-Cabello M, Campos A, Vasconcelos V, et al. New method for simultaneous determination of microcystins and cylindrospermopsin in vegetable matrices by SPE-UPLC-MS/MS. Toxins. 2018;10:406.
Acknowledgements
The authors would like to acknowledge the funding from the research office at Alfaisal University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving humans and animal rights
This research did not involve human participants and/or animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chinnappan, R., AlZabn, R., Fataftah, A.K. et al. Probing high-affinity aptamer binding region and development of aptasensor platform for the detection of cylindrospermopsin. Anal Bioanal Chem 412, 4691–4701 (2020). https://doi.org/10.1007/s00216-020-02723-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02723-4