Abstract
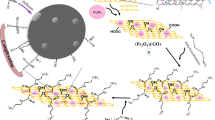
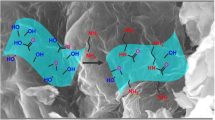
The aim of the present study was to develop a novel adsorbent through decorating the surface of graphene oxide (GO) nanosheets with nickel ferrite (NiFe2O4) magnetic particles and subsequent functionalized with an eco-friendly and biologically active tripeptide, glutathione (GSH). Diverse characterization techniques including FE–SEM, EDX, XRD, FT–IR, VSM and BET along with the pHpzc measurements were employed to analyze the features of GSH-NiFe2O4/GO composite in detail. The efficacy of the composite for adsorption of three toxic heavy metal ions including Hg(II), Cu(II), and Pb(II) was assessed in aqueous media, individually. The effects of solution pH, operating time, initial metal ion concentration on capturing metal ions by GSH-NiFe2O4 were systematically studied in batch systems. The adsorption of metal ions was severely pH-dependent. Notably, the removal efficiencies of higher than 94% was obtained at pH 5.0 and 6.0 from 20 mg L−1 single solution containing Pb(II) and both Hg(II) and Cu(II) ions, in the respective order. The adsorption kinetics was nicely fitted by the pseudo-second-order and Elovich rate equations, whereas Freundlich isotherm equation described well the equilibrium adsorption data. The maximum adsorption capacity was found to be 272.94, 266.22, and 204.06 mg g−1 for Hg(II), Cu(II), and Pb(II) at 298 K. The experiments in binary solutions containing interfering ions clarified that prepared composite has higher selectivity toward Hg(II) than Pb(II) or Cu(II). Moreover, the Zn(II) and Fe(II) cations showed negligible competitive adsorption with all three target ions. The findings also demonstrated that GSH-NiFe2O4 has a prominent performance to decontaminate the groundwater samples spiked with the aforementioned metal ions.










Similar content being viewed by others
References
H. Ansari, M. Miralinaghi, F. Azizinezhad, Cell. Chem. Technol. Cell Chem. Technol. 53, 191 (2019)
Q. Zhang, N. Liu, Y. Cao, W. Zhang, Y. Wei, L. Feng, L. Jiang, Appl. Surf. Sci. 434, 57 (2018)
C. Rizzo, J.L. Andrews, J.W. Steed, F. D’Anna, J. Colloid Interface Sci. 548, 184 (2019)
Q.-U. Ain, M.U. Farooq, M.I. Jalees, J. Water Process Eng. 33, 101044 (2020)
A.E. Burakov, E.V. Galunin, I.V. Burakova, A.E. Kucherova, S. Agarwal, A.G. Tkachev, V.K. Gupta, Ecotoxicol. Environ. Saf. 148, 702 (2018)
W. Wang, K. Cai, X. Wu, X. Shao, X. Yang, J. Alloys Compd. 722, 532 (2017)
A.A. Tabatabaiee Bafrooee, H. Ahmad Panahi, E. Moniri, M. Miralinaghi, A.H. Hasani, Environ. Sci. Pollut. Res. 27, 9547 (2020)
S.Y. Mirnezami, M. Davallo, M. Sohrabi, F. Motiee, M. Khosravi, Res. Chem. Intermed. 45, 5519 (2019)
J. Yang, Y. Chu, Z. Li, Y. Zhang, Environ. Sci. Pollut. Res. 25, 33464 (2018)
A. Homayonfard, M. Miralinaghi, R.H.S.M. Shirazi, E. Moniri, Water Sci. Technol. 78, 2297 (2018)
Y. Guo, J. Deng, J. Zhu, X. Zhou, R. Bai, RSC Adv. 6, 82523 (2016)
L.G. Bach, T. Van Tran, T.D. Nguyen, T. Van Pham, S.T. Do, Res. Chem. Intermed. 44, 1661 (2018)
G.K. Sarma, S. Sen Gupta, K.G. Bhattacharyya, Environ. Sci. Pollut. Res. 26, 6245 (2019)
C. Santhosh, P. Kollu, S. Felix, V. Velmurugan, S.K. Jeong, A.N. Grace, RSC Adv. 5, 28965 (2015)
K.K. Kefeni, B.B. Mamba, T.A.M. Msagati, Sep. Purif. Technol. 188, 399 (2017)
H.-Y. Zhu, R. Jiang, S.-H. Huang, J. Yao, F.-Q. Fu, J.-B. Li, Ceram. Int. 41, 11625 (2015)
B. Xiang, D. Ling, H. Lou, H. Gu, J. Hazard. Mater. 325, 178 (2017)
R. Ahmad, A. Mirza, Groundw Sustain. Dev. 7, 305 (2018)
X. Mao, L. Wang, C. Wang, E. Lichtfouse, Environ. Chem. Lett. 16, 1429 (2018)
P. Xu, G.M. Zeng, D.L. Huang, M. Yan, M. Chen, C. Lai, H. Jiang, H.P. Wu, G.M. Chen, J. Wan, J. Taiwan Inst. Chem. Eng. 71, 165 (2017)
W.S. Hummers, R.E. Offeman, J. Am. Chem. Soc. 80, 1339 (1958)
R. Seenivasan, W.-J. Chang, S. Gunasekaran, ACS Appl. Mater. Interfaces. 7, 15935 (2015)
A. Banazadeh, S. Mozaffari, B. Osoli, J. Environ. Chem. Eng. 3, 2801 (2015)
T. Ahmad, H. Bae, Y. Iqbal, I. Rhee, S. Hong, Y. Chang, J. Lee, D. Sohn, J. Magn. Magn. Mater. 381, 151 (2015)
A. Santhana Krishna Kumar, S.-J. Jiang, RSC Adv. 5, 6294 (2015)
J.I. Langford, A.J.C. Wilson, J. Appl. Crystallogr. 11, 102 (1978)
L.P. Lingamdinne, Y.L. Choi, I.S. Kim, J.K. Yang, J.R. Koduru, Y.Y. Chang, J. Hazard. Mater. 326, 145 (2017)
P. Miralinaghi, P. Kashani, E. Moniri, M. Miralinaghi, Mater. Res. Express 6, 065305 (2019)
N. Ballav, R. Das, S. Giri, A.M. Muliwa, K. Pillay, A. Maity, Chem. Eng. J. 345, 621 (2018)
S. Banerjee, Res. Chem. Intermed. 1 (2018)
S.M. Najdanović, M.M. Petrović, M.M. Kostić, J.Z. Mitrović, D.V. Bojić, M.D. Antonijević, A.L. Bojić, Res. Chem. Intermed. (2019)
S.E. Rokni, R. Haji Seyed Mohammad Shirazi, M. Miralinaghi, E. Moniri, Res. Chem. Intermed. 46, 2247 (2020)
M.R. Samarghandi, M. Khiadani, M. Foroughi, H. Zolghadr Nasab, Environ. Sci. Pollut. Res. 23, 887 (2016)
X. Tao, K. Li, H. Yan, H. Yang, A. Li, Environ. Pollut. 209, 21 (2016)
A. Shahzad, W. Miran, K. Rasool, M. Nawaz, J. Jang, S.R. Lim, D.S. Lee, RSC Adv. 7, 9764 (2017)
X. Li, S. Wang, Y. Liu, L. Jiang, B. Song, M. Li, G. Zeng, X. Tan, X. Cai, Y. Ding, J. Chem. Eng. Data 62, 407 (2017)
Y. Zhang, Y. Liu, X. Wang, Z. Sun, J. Ma, T. Wu, F. Xing, J. Gao, Carbohydr. Polym. 101, 392 (2014)
L. Cui, Y. Wang, L. Gao, L. Hu, L. Yan, Q. Wei, B. Du, Chem. Eng. J. 281, 1 (2015)
F. Einollahi Peer, N. Bahramifar, H. Younesi, J. Taiwan Inst. Chem. Eng. 87, 225 (2018)
D. Allouss, Y. Essamlali, A. Chakir, S. Khadhar, M. Zahouily, Environ. Sci. Pollut. Res. 27, 7476 (2019)
A. Moradi, P. Najafi Moghadam, R. Hasanzadeh, M. Sillanpää, RSC Adv. 7, 433 (2017)
R.D.C. Soltani, G.S. Khorramabadi, A.R. Khataee, S. Jorfi, J. Taiwan Inst. Chem. Eng. 45, 973 (2014)
Z. Sekhavat Pour, M. Ghaemy, RSC Adv. 5, 64106 (2015)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Khorshidi, P., Shirazi, R.H.S.M., Miralinaghi, M. et al. Adsorptive removal of mercury (II), copper (II), and lead (II) ions from aqueous solutions using glutathione-functionalized NiFe2O4/graphene oxide composite. Res Chem Intermed 46, 3607–3627 (2020). https://doi.org/10.1007/s11164-020-04164-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04164-1