Abstract
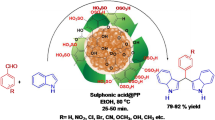
α,α′-bis(substituted benzylidene)cycloalkanones were efficiently prepared from variously substituted aldehydes and cycloalkanones in water by using ash of pomegranate peels (APP) as a catalyst. The APP-catalyst was obtained from bio-waste by simple thermal treatment to dry peels of pomegranate fruit and formation of its active phase was confirmed by FT-IR, XRD, XRF, EDX, SEM, DSC-TGA and BET techniques. The analysis revealed that the present catalyst has basic sites which promote the synthesis of desired products. The main attractions of our protocol are utilization of highly abundant bio-waste-derived catalyst and good-to-excellent yield in shortest reaction time. This green protocol was further extended for structurally diverse 2-arylidene-1-tetralones by condensation of equimolar quantity of aromatic aldehydes and 1-tetralone at low temperature. The catalyst could be quantitatively recovered and reused effectively for five times.
Graphic abstract














Similar content being viewed by others
References
L. Zhang, S. Wang, E. Sheng, S. Zhou, Green Chem. 7, 683 (2005)
P. Anastas, J. Warner, Green Chemistry: Theory and Practice, vol. 30 (Oxford University Press, New York, 1998)
M.B. Gawande, V.D.B. Bonifacio, R. Luque, P.S. Branco, R.S. Varma, Chem. Soc. Rev. 42, 5522 (2013)
Y. Wu, J. Hou, Y. Liu, M. Zhang, C. Tung, Y. Wang, Tetrahedron 72(12), 1511 (2016)
S.Z.D. Heirati, F. Shirini, A.F. Shojaei, Res. Chem. Intermed. 43, 6167 (2017)
D. Song, Y. Chen, R. Wang, C. Liu, H. Jiang, G. Luo, Prep. Biochem. Biotech. 39, 201 (2009)
J. Deli, T. Lonard, D. Szabo, A. Foldesi, Pharmazie 39, 539 (1984)
A.T. Dinkova-Kostova, C. Abeygunawardana, P. Talalay, J. Med. Chem. 41, 5287 (1998)
C. Piantadosi, I.H. Hall, J.L. Irvine, G.L. Carlson, J. Med. Chem. 16, 770 (1973)
S.F.P. Braga, E.V.P. Alves, R.S. Ferreira, J.R.B. Fradico, P.S. Lage, M.C. Duarte, T.G. Ribeiro, P.A.S. Junior, A.J. Romanha, M.L. Tonini, M. Steindel, E.F. Coelho, R.B. de Oliveira, Eur. J. Med. Chem. 71, 282 (2014)
A.A. Raj, R. Raghunathan, M.R. Sridevi Kumari, N. Raman, Bioorg. Med. Chem. 11, 407 (2003)
J.R. Dimmock, N.W. Hamon, K.W. Hindmarsh, A.P. Sellar, A.W. Turner, G.H. Rank, A.J. Robertson, J. Pharm. Sci. 65, 538 (1976)
R. Costi, R.D. Santo, M. Artico, G. Miele, P. Valentini, E. Novellino, A. Cereseto, J. Med. Chem. 50, 1973 (2007)
J.X. Wang, L. Kang, Y. Hu, B. Wei, Synth. Commun. 32(11), 1691 (2002)
J. Li, W. Yang, G. Chen, T. Li, Synth. Commun. 33, 2619 (2003)
T. Hosoya, A. Nakata, F. Yamasaki, F. Abas, K. Shaari, N. Lajis, H. Morita, J. Nat. Med. 66, 166 (2012)
S. Javanshir, M.M. Mojtahedi, J. Eslami, Curr. Chem. Lett. 3, 63 (2014)
M. Vashishtha, M. Mishra, S. Undre, M. Singh, D.O. Shah, J. Mol. Catal. A Chem. 396, 143 (2015)
H. Karimzadegan, B. Akhlaghinia, M.S. Ghasemzadeh, Iran. J. Catal. 9(2), 109 (2019)
M.S. Abaee, M.M. Mojtahedi, R. Sharifi, M.M. Zahedi, H. Abbasi, K. Tabar-Heidar, J. Iran. Chem. Soc. 3(3), 293 (2006)
L.T. An, J.P. Zou, L.L. Zhang, Catal. Communi. 9, 349 (2008)
U.P. Patil, R.C. Patil, S.S. Patil, React. Kinet. Mech. Catal. 129, 679 (2020)
M.B. Deshmukh, S.S. Patil, S.D. Jadhav, P.B. Pawar, Synth. Commun. 42, 1177 (2012)
S.S. Patil, S.D. Jadhav, M.B. Deshmukh, J. Chem. Sci. 125, 851 (2013)
S.K. Shinde, S.A. Damate, S.T. Morbale, M.U. Patil, S.S. Patil, RSC Adv. 7, 7315 (2017)
S.K. Shinde, M.U. Patil, S.A. Damate, S.S. Patil, Res. Chem. Intermed. 44(3), 1775 (2018)
U.P. Patil, R.C. Patil, S.S. Patil, J. Heterocycl. Chem. 56, 1898 (2019)
S.R. Mali, S.K. Shinde, S.A. Damate, S.S. Patil, R. Soc, Open Sci. 5, 170333 (2018)
S.T. Morbale, S.K. Shinde, S.A. Damate, M.B. Deshmukh, S.S. Patil, Lett. Org. Chem. 15(1), 57 (2017)
S.T. Morbale, S.D. Jadhav, M.B. Deshmukh, S.S. Patil, RSC Adv. 5, 84610 (2015)
R.M. Appa, S.S. Prasad, J. Lakshmidevi, B.R. Naidu, M. Narasimhulu, K. Venkateswarlu, Appl. Organometal Chem. (2019)
J. Lakshmidevi, R.M. Appa, B.R. Naidu, S.S. Prasad, L.S. Sarma, K. Venkateswarlu, Chem. Commun. 54, 12333 (2018)
P.B. Hiremath, K. Kamanna, Curr. Microw. Chem. 6(1), 30 (2019)
E. Tabrizian, A. Amoozadeh, S. Rahmani, M. Salehi, M. Kubicki, Res. Chem. Intermed. 42(2), 531 (2015)
G.H. Mahdavinia, S. Rostamizadeh, A.M. Amani, M. Mirzazadeh, Green. Chem. Lett. Rev. 5(3), 255 (2012)
C.Y. Zhao, J.Y. Liu, Y. Wang, X.J. Zhao, B. Yuan, M.M. Yue, Synth. Commun. 1(44), 827 (2014)
R. Pal, IOSR-JAC 3(4), 74 (2013)
S. Ahmadi, A. Zare, M. Aali-Hosaini, M. Maghsoudi, S. Izadpanah, A. Parhami, M. Merajoddin, Res. Chem. Intermed. 42(7), 6245 (2016)
W.M. Welch, C.A. Harbert, R. Sarges, W.P. Stratten, A. Weissman, J. Med. Chem. 20(5), 699 (1977)
T.A. Nakibl, V. Bezjakl, M.J. Meeganz, R. Chandy, Eur. J. Med. Chem. 25, 455 (1990)
D.F. Biggs, A.F. Casy, I. Chu, R.T. Coutts, Eur. J. Med. Chem. 19, 472 (1976)
J.R. Dimmock, M.P. Padmanilyam, G.A. Zello, J.W. Quail, E.O. Oloo, J.S. Prisciak, H.B. Kraatz, A. Cherkasov, J.S. Lee, T.M. Allen, C.L. Santos, E.K. Manavathu, E. De Clercq, J. Balzarini, J.P. Stables, Eur. J. Med. Chem. 37, 813 (2002)
A.S. Aboraia, B. Makowski, A. Bahja, D. Prosser, A. Brancale, G. Jones, C. Simons, Eur. J. Med. Chem. 45, 4427 (2010)
V.P. Kumar, J. Renjitha, C.T. Fathimath Salfeena, K.T. Ashitha, S.K. Rangappa, V. Sunil, B.S. Sasidhar, Chem. Biol. Drug. Des. 90, 703 (2017)
S.K. Mandal, A. Sarkar, J. Chem. Soc. Perkin Trans. 1, 669 (2002)
B. Hallgas, Z. Dobos, E. Osz, F. Hollosy, R.E. Schwab, E.Z. Szabo, D. Eros, M. Idei, G. Keri, T. Lorand, J. Chromatogr. B 819, 283 (2005)
A. Sultan, A.R. Raza, M. Abbas, K.M. Khan, M.N. Tahir, N. Saari, Molecules 18, 10081 (2013)
T.M. Al-Nakib, T.L. Andras, F.R. Varghese, Med. Princ. Pract. 10, 191 (2001)
R. Kaur, M. Bansal, B. Kaur, Chem. Sci. J. 18, 1118 (2011)
S. Arora, A. Pareek, N. Agrawal, B.P. Nagori, IJRPC 3(4), 797 (2013)
R. Kamakshi, S. Swarna Latha, B.S.R. Reddy, Indian J. Chem. 49B, 944 (2010)
V. Tomeckova, J. Guzy, J. Kušnir, K. Fodor, M. Marekova, Z. Chavkova, P. Perjesi, J. Biochem. Biophys. Methods 69, 143 (2006)
B. Das, P. Thirupathi, I. Mahender, K.R. Reddy, J. Mol. Catal. A Chem. 247, 182 (2006)
J.J. Shrikhande, M.B. Gawande, R.V. Jayaram, Catal. Commun. 9, 1010 (2008)
B. Mounir, F. Bazi, A. Mounir, H. Toufik, M. Zahouily, Green Sustain. Chem. 8, 156 (2018)
T.S. Jin, Y. Zhao, L.B. Liu, T.S. Li, Indian J. Chem. 45B, 1965 (2006)
Acknowledgements
Authors are thankful to Indian Institute of Chemical Technology (IICT), Hyderabad for NMR analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patil, R.C., Patil, U.P., Jagdale, A.A. et al. Ash of pomegranate peels (APP): A bio-waste heterogeneous catalyst for sustainable synthesis of α,α′-bis(substituted benzylidine)cycloalkanones and 2-arylidene-1-tetralones. Res Chem Intermed 46, 3527–3543 (2020). https://doi.org/10.1007/s11164-020-04160-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04160-5