Abstract
The effects of the amphoteric and basic oxides alumina and lime on the phase equilibria of copper matte and silica-saturated slags were investigated at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm in a controlled CO-CO2-SO2-Ar gas atmosphere using a high-temperature isothermal equilibration technique followed by rapid quenching. The equilibrium phase compositions were obtained by Electron Probe X-ray Microanalysis. The relationship between the copper concentration in matte and the oxygen partial pressure, iron, and sulfur in matte was quantified. The pure iron-silicate slag exhibited the highest copper loss in slag, although the addition of alumina and lime decreased its value by approximately a quarter and a half, respectively, at a matte grade of 65 wt pct Cu. In contrast, copper and sulfur were highly distributed in the matte phase, and their deportment to the matte was favored by addition of alumina and lime.
Similar content being viewed by others
Introduction
The flash smelting technology developed by Outokumpu in 1949 in Harjavalta (Finland) is regarded as one of the most important innovations in metallurgy in the 20th century.[1] Copper sulfide concentrates are introduced into flash smelter with the aim of utilizing the energy contained in the sulfide minerals,[2] producing copper, oxide slag, and sulfur dioxide gas. As more complex mineral ores and secondary raw materials are increasingly treated in copper smelting,[3,4] impurities like Al2O3 will be introduced into the smelting system with the addition of WEEE and fluxes. As a result, copper smelting is becoming more complicated and involves more metals of economic value. Accurate fundamental thermodynamic phase equilibrium information as well as optimal control of slag chemistry are thus of great interest for efficient and stable operation.
The matte/slag/tridymite equilibria have been widely studied for the Cu-Fe-S-O-SiO2 system[5,6,7,8,9,10] over the past few decades. The discrepancies between those studies can be ascribed to the experimental techniques and analytical methods used. Use of large samples[5,6,7,8,9] incurs difficulties in reaching equilibrium between gas and condensed phases. Wet chemical analyses[5,6,7,8,9] after physical separation of matte and slag contribute to the values and uncertainties of the phase compositions. Moreover, the considerable scatter, probably due to non-detected inhomogeneities, in the previous studies make it difficult to derive detailed conclusions on the phase relations and metal distributions.
Recently, Abdeyazdan et al.[11] and Fallah-Mehrjardi et al.[12,13,14,15] studied the slag/matte/tridymite equilibria in the Cu-Fe-S-O-SiO2 system at 1200 to 1300 °C[10] and \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 to 0.25 atm using a high-temperature equilibration and quenching technique in flowing CO-CO2-SO2-Ar gas. The phase compositions of matte and slag were analyzed by EPMA (Electron Probe X-ray Microanalysis). They also investigated the effects of MgO and CaO on slag/matte/tridymite equilibria.[11,16] They reported that the dissolved copper and sulfur in slag were strongly affected by the MgO and CaO concentrations, and that the Fe/SiO2 ratio in slags decreased with increasing MgO and CaO concentrations.[11,16]
Avarmaa et al.[17,18] and Sukhomlinov et al.[19] investigated the phase equilibria of copper matte with trace elements and silica-saturated iron silicate slags at 1250 to 1350 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm, by a high-temperature equilibration/quenching/EPMA and Laser Ablation-Inductively Coupled Plasma-Mass Spectrometry (LA-ICP-MS) technique. It was found that the copper loss in the slag was increased by increasing the temperature[17,18] and matte grade.[17,18,19] Addition of Al2O3 and CaO effectively decreased the copper loss and sulfur concentration in the slag but had no detectable effect on the concentration of iron and sulfur in the matte.[19]
The most recent systematic research on the effects of the basic oxides Al2O3, CaO, and MgO on the phase equilibrium of copper matte and slag at tridymite saturation was conducted by Shishin et al.[20] at 1200 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.25 atm, using both experimental and thermodynamic modeling. They suggested that the Fe/SiO2 ratio as well as the sulfur and copper concentrations in slag decreased with increasing Al2O3, CaO, and MgO concentrations. However, the matte composition was not strongly affected by addition of basic oxides.
Despite extensive studies on the phase equilibria of the Cu-Fe-S-O-SiO2 system, only few experimental studies exist on the effects of basic oxides on the matte/slag/tridymite equilibria. The present study was focused on the equilibrium phase relations of copper matte and three different silica-saturated slags: pure iron silicate, iron silicate with 10 wt pct Al2O3, and iron silicate with 10 wt pct Al2O3 and 10 wt pct CaO, at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm related to pyrometallurgical WEEE processing through the copper smelting with air blowing. The detailed distributions of precious metals in the same experiments were reported in our previous study.[21]
Experimental
Analytical high purity powders were employed for synthesizing the copper matte and slag in situ. The materials used are listed in Table I. The starting copper matte mixtures were prepared with the composition of Cu2S/FeS = 70/30 (w/w) for target matte grades of 55 to 70 wt pct Cu, and Cu2S/FeS = 80/20 for a matte grade of 75 wt pct Cu. The slag mixtures were prepared in weight ratios of Fe2O3/SiO2 = 70/30, Fe2O3/SiO2/Al2O3 = 47/43/10, and Fe2O3/SiO2/Al2O3/CaO = 24/56/10/10 with initial Fe/SiO2 ratios of 1.63, 0.76, and 0.30, respectively. The initial slag compositions were designed to follow the tridymite-slag phase boundary of the FeOx-SiO2-Al2O3 and FeOx-SiO2-Al2O3-CaO systems in equilibrium gas atmosphere.[21] Approximately 0.1 g of copper matte was equilibrated with an equal amount of slag in each experiment. Bowl-shaped, fused silica crucibles with dimensions of 6/10 mm (H/OD) were used to hold matte-slag pellets that had been compacted by a hydraulic press. The use of silica support ensured silica saturation in the matte-slag system and avoided possible contamination of the condensed phases by the crucible material. The partial pressures of SO2, S2, and O2 in the furnace were controlled by a flowing gas mixture of CO (99.99 vol pct), CO2 (99.999 vol pct), SO2 (99.99 vol pct), and Ar (99.999 vol pct). In all experiments, SO2 partial pressure was fixed to 0.1 atm. All gases used in this study were from AGA-Linde (Finland),[21] regulated by DFC26 digital mass-flow controllers (Aalborg, USA), and introduced into the furnace after being premixed in a guiding pipe. The gas atmospheres calculated with MTDATA thermodynamic software[22] using the SGTE pure substance database[23] for target matte grades are listed in Table II. The values of \( P_{{{\text{O}}_{ 2} }} \) and \( P_{{{\text{S}}_{ 2} }} \) were calculated based on the equilibrium constants of reactions [1] through [2].
The experimental methodology used in this study was similar to that described in our previous studies.[17,18,19,21] It involved high-temperature equilibration on a silica substrate in a vertical tube furnace (Lenton PTF 15/45/450), rapid quenching of the equilibrated samples in ice-water mixtures, wet-metallographic preparation of samples, and direct phase composition analysis by EPMA. The experimental furnace consisted of silicon carbide heating elements and an impervious recrystallized alumina reaction tube. The Eurotherm 3216 PID controllers enabled three-zone temperature control of the furnace. The sample temperature was measured by a calibrated S-type Pt/90 pct Pt-10 pct Rh thermocouple (Johnson-Matthey Noble Metals, UK), located next to the sample. The thermocouple was connected to a 2010 DMM multimeter (Keithley, USA). A Pt100 resistance thermometer (SKS Group, Finland) connected to a 2000 DMM multimeter (Keithley, USA) measured the cold junction temperature. A schematic diagram of the experimental furnace is shown in Figure 1.
A time series was carried out for equilibration by annealing the matte/pure iron-silicate slag pellets at 1300 °C for 2, 4, and 6 h at \( P_{{{\text{O}}_{ 2} }} \) = 10−8.1 and 10−7.6 atm. The time needed for equilibration was defined according to the concentrations of SiO2 in the slag and Cu in the matte (Figure 2). The compositions obtained in the time series were measured by Tescan MIRA 3 Scanning Electron Microscope (SEM, Tescan, Brno, Czech Republic) equipped with an UltraDry Silicon Drift Energy Dispersive X-ray spectrometer (EDS, Thermo Fisher Scientific, Waltham, MA, USA) and NSS Microanalysis software. Based on the results, the equilibration time required to attain uniform and constant phase compositions in both matte and slag was defined to be four hours.
The samples for microanalysis were prepared by cutting the crucibles into half and mounting them in epoxy resin (EpoFix, Struers, Denmark). The cross-sections were ground and polished. The polished sections were carbon coated with a LEICA EM SCD050 sputtering device (Leica Microsystems, Austria) to ensure sufficient electrical conductivity. The microstructures and elemental compositions of the condensed phases were pre-examined by SEM-EDS. The direct measurements of chemical compositions of matte and slag were conducted with a Cameca SX100 Electron Microprobe (Cameca SAS, Genevilliers, France) equipped with five Wavelength Dispersive Spectrometers (WDS) using an accelerating voltage of 20 kV, a beam current of 60 nA, and a beam diameter of 100 µm for matte and 50 to 100 µm for slag. The analysis areas in the copper matte and slag were chosen from well-quenched, homogeneous regions without larger segregations. The reported average analyses of each phase consisted of eight points to minimize the scatter and uncertainty. The standards employed for EPMA and the elemental detection limits are listed in Table III. A PAP-ZAF on-line matrix correction program [24] was employed for the raw data processing before normalizing the assays.
Results and Discussion
Microstructures of the Copper Matte and Slag
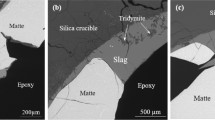
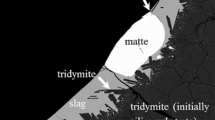
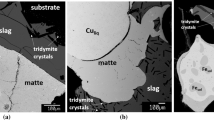
Typical microstructures of the matte-slag systems equilibrated at \( P_{{{\text{O}}_{ 2} }} = 10^{ - 8.0} \) atm for a target matte grade of 60 wt pct Cu are illustrated in Figure 3. It can be confirmed that copper matte and slag were in equilibrium with tridymite in all samples. The rod-like crystals in the slag are secondary tridymite due to the dissolution of the silica crucible and an excess of silica in the initial slag mixtures. Comparison of the microstructures obtained with different slags indicates that the FeOx-SiO2 slag was more aggressive, as wider cracks were formed in the silica crucible, resulting in an intensive penetration of molten slag into the crucible wall. With addition of alumina and lime, the formation of secondary tridymite crystals was reduced.
Matte Composition
The obtained constituents of copper matte plotted against different variables are shown in Figure 4. The available data from previous studies[17,19] were also plotted in the graph for comparison.
Figure 4(a) presents the experimental matte grade as a function of logarithmic oxygen partial pressure. The matte grade increased with increasing oxygen partial pressure, but the influence of oxygen partial pressure on the matte grade gradually decreased at higher \( P_{{{\text{O}}_{ 2} }} \). The addition of alumina and lime into the slags at silica saturation led to an increase of matte grade under the same experimental conditions. The impact of the slag modifiers on the matte grade decreased towards white metal (Cu2S) compositions. The observed trends are related to reaction [3], as suggested by Yazawa,[25] and reaction [4] given by Fallah-Mehrjardi et al.[15] for matte and slag in equilibrium with gaseous sulfur, oxygen, and sulfur dioxide. The addition of alumina and lime into silica-saturated iron silicate slags decreased the FeO activity and a subsequent decrease of iron activity in the matte resulted in an increase of the matte grade.
Figure 4(b) shows the site fraction of copper in the metal sub-lattice of the matte, equilibrated with different types of silica-saturated slags. Different slags are located on the same straight line and decreased with the increasing iron concentration of the matte, indicating that the slag composition had no impact on the copper concentration in the metal sub-lattice of matte. The site fraction of copper in the metal sub-lattice of the matte y(Cu) was calculated using Eq. [5]:
where n is the molar composition defined as n(X) = w(X)/N(X) and N(X) is the molar mass of metal X. In addition, it can be observed in Figure 4(b) that the copper site fractions in Sukhomlinov’s study[17] are slightly on the higher side than in the present study, whereas the results by Avarmaa et al.[17] are located on the lower side, due to noble elements dissolved in the matte. Similar deviations were also observed for iron and sulfur concentrations in the matte, as shown in Figures 4(c) and (d), respectively. As for the iron and sulfur concentrations in the matte, they showed similar downward trends as a function of matte grade in the reference studies.[11,13,14,15,16,17,19,20] The iron and sulfur concentrations in the matte were not affected by the addition of alumina and lime into the iron silicate slag, which agrees well with the observations made by Sukhomlinov et al.[19] and Shishin et al.[20]
In order to clarify the reasons behind the differences between the present study and some recent observations in the literature,[17,19] total concentrations of the trace elements in the matte phase were plotted as a function of the matte grade, see Figure 4(e). In the study by Avarmaa et al.,[17] in addition to the same trace elements as those used in the present study, 1 wt pct rhodium was also added into the copper matte. In the study by Sukhomlinov et al.,[19] gallium, indium, iridium, tin, tellurium, and molybdenum were introduced into the matte-slag system but they were largely deported in the slag or vaporized. As indicated in Figure 4(e), the total concentration of trace elements in the present study was lower than in Avarmaa et al.[17] but higher than in Sukhomlinov et al.[19] Therefore, the differences in copper site fractions in the metal sub-lattice of the matte as well as in the iron and sulfur concentrations in the matte can be ascribed to differences in its trace element concentrations deported in the sulfide matte.
Slag Composition
The slag compositions with experimental standard deviations as a function of matte grade or silica concentration are illustrated in Figure 5. The copper loss in slags is of particular industrial interest due to its importance in slag cleaning for recovering the copper values.
The copper concentration in the slag obtained in the present study indicates that the copper loss increased with increasing matte grade. It is, however, evident that the alumina and lime modifications can effectively decrease copper loss in the slags, as reported in the literature.[19,20,26,27] As a principle, silicate slag is able to sustain complex polymeric silicate compounds, which leads to a high slag viscosity. However, the three-dimensional polymeric silicate network dissociates upon the addition of basic oxide,[28,29] which results in a decrease of slag viscosity and mechanical copper losses. As for the decrease in chemical copper loss from addition of basic oxide, Mackey et al.[28] proposed that the copper cations in slags were being replaced by Al3+, Mg2+, and Ca2+ ions. The increasing impact order of Al2O3 and CaO on decreasing the copper loss obtained in the present study is consistent with that of the basicity of the additives, supporting the acid-base theory of the slags.[30,31,32]
The copper loss in slag has been intensively investigated in previous studies[6,11,15,16,17,33,34,35,36,37,38] because of its great significance for copper resource efficiency. Results from other studies[6,11,15,16,17,33,34,35,36,37,38] are summarized in Figure 5(b) for comparison. The experimental conditions used in those studies are listed in Table IV. The previously measured copper solubility data are quite scattered, but all the trend lines for copper increased with increasing matte grade, except for the results in spinel-saturated FeOx-SiO2 slags, which show the opposite, a decreasing trend. The data for the SiO2-saturated FeOx-SiO2 slag in the present study agrees well with the results by Avarmaa et al.[17] Some researchers[8,25,39,40,41] adopted the concept of sulfidic and oxidic copper dissolution in slags to understand the mechanism and nature of the total chemical dissolved copper loss. A similar oxidic/sulfidic dissolution concept has also been applied to express the nickel[42] and lead[43] loss in slags. Although the concept is useful for evaluating the effect of sulfur on the solubility of copper, it is not sufficient to explain the overall behavior of sulfur and copper in slags. Hence, further effort is needed to investigate the copper dissolution mechanism in slags in the copper smelting process.
Figure 5(c) indicates that pure silica-saturated iron-silicate slag exhibits the highest sulfur concentration in slag and that it decreased with increasing matte grade. Addition of alumina and lime into the slags can effectively suppress the sulfur dissolution, as also observed by Sukhomlinov et al.[19] and many others.[16,20] Shimpo et al.[9] proposed that the sulfur in the slag was mainly combined with iron, i.e., FeS, due to the affinity of the Fe2+ ion for sulfur, whereas the addition of alumina and lime decreased the content of the Fe2+ ion and, consequently, the slag lost part of its ability to dissolve sulfur.[9] Although the Fe2+ content was not measured in our study, the study by Shimpo et al.[9] can provide some guidance for further study on the existing form of sulfur in slag equilibrated with sulfide copper matte.
As for the silica concentration and Fe/SiO2 ratio, the results obtained for the pure iron-silicate slags of the present study agree well with the literature.[19] The results with alumina- and lime-containing slags have a similar trend as that reported by Sukhomlinov et al.,[19] although the alumina and lime concentrations are different. Figure 5(d) shows that silica concentration in the alumina- and lime-free slag at silica saturation increased from 33 to approximately 36 wt pct over the matte grade range investigated. The corresponding Fe/SiO2 ratio shown in Figure 5(e) decreased from 1.45 to 1.35, whereas the ratio of the other two slags showed constant values as a function of the matte grade.
Alumina and lime concentrations in the silica-saturated slags, as a function of the silica concentration, are shown in Figure 5(f) along with the reference study.[19] The concentrations of alumina and lime differed between the studies. However, they have general similar increasing trends with increasing silica concentration in slags, showing that the addition of alumina and lime to the iron silicate slags increases the silica solubility in the slags.[9,44,45]
Phase Diagrams
Figure 6 shows the quasi-binary phase diagrams of the FeOx-SiO2-Al2O3 and FeOx-SiO2-Al2O3-8 wt pct CaO systems predicted by MTDATA with its MTOX database[22] over the alumina concentration range of 0 to 20 wt pct. As shown in Figure 6(a), the liquidus temperature of the FeOx-SiO2-Al2O3 system decreased with increasing alumina concentration up to about 8 wt pct, after which it started to increase. However, in the lime-containing slag system, a small amount of added alumina, approximately higher than 0.7 wt pct, would lead to an increase in the liquidus temperature. The effect of lime on the phase equilibria and liquidus temperature of the slag can be observed by comparing Figures 6(a) and (b).
To demonstrate the range of the slag compositions and phase relations of the FeOx-SiO2-Al2O3 and FeOx-SiO2-Al2O3-8 wt pct CaO systems, the isothermal sections of the superimposed phase diagram predicted by MTDATA are shown in Figure 7. In both systems, the extent of the molten liquid slag domain was limited by the formation of either tridymite or spinel, depending on the Fe/SiO2 ratio. The maximum solubility of Al2O3 in the lime-free and lime-containing slag was limited by the formation of mullite or feldspar (CaAl2Si2O8), respectively. The addition of lime to the iron silicate slag increased both the molten slag primary phase field and the silica concentration on the silica-saturation line, and it also decreased the liquidus temperature. Comparison of the solid tridymite-molten slag phase boundary between the experimentally measured points and the lines predicted by MTDATA are also displayed in Figure 7. All the experimental data listed in Table V are averaged results of independent analysis points for the slag phase. The predicted phase boundary of the FeOx-SiO2-Al2O3 system agrees well with the experimental data, whereas the results for the FeOx-SiO2 and FeOx-SiO2-Al2O3-CaO systems have small discrepancies with the calculated data.
An isothermal section of the FeOx-SiO2-Al2O3 (green line) and FeOx-SiO2-Al2O3-8 wt pct CaO (black line) systems at 1300 °C and \( P_{{{\text{O}}_{ 2} }} \) = 10−8 atm; OX_LIQ-molten slag; G-gas; TRI-tridymite; SP-spinel; FSP-feldspar; MUL-mullite; ⊡ CaO-free slags and ◯ the Al2O3-CaO modified slags of this study (Color figure online)
Distributions of Copper, Iron, and Sulfur Between Copper Matte and Slag
The distributions of elements between copper matte and slag determine the efficiency of recovery and accumulation of valuable metals in the sulfide matte or, alternatively, how efficiently impurities are removed to the slag. The distribution coefficients of copper, iron, and sulfur between copper matte and slag were defined as the weight ratio of the element concentrations in the matte and slag, as formulated in the following Eq. [6]:
where [wt pct Me] refers to the experimentally measured average concentration in matte, and (wt pct Me) refers to the corresponding concentration in slag. The obtained distribution coefficients with their uncertainties shown as error bars are illustrated in Figure 8 as a function of the matte grade.
Figure 8(a) indicates that the deportment of copper to the matte phase was highly favored by the addition of alumina and lime; its distribution into matte equilibrated with alumina- and lime-containing slags decreased with an increasing matte grade. The distribution coefficient of copper between the matte and pure iron-silicate slags stayed relatively constant at between 60 and 70, whereas addition of alumina and alumina-lime increased its value up to 90 and 150 respectively at 65 wt pct Cu matte. However, the behavior of precious metals between copper matte and slag have opposite trends as a function of increasing matte grade, i.e., higher matte grades can effectively increase the recoveries of precious metals, as indicated in previous studies.[17,18,19]
The trend lines for the distribution coefficients of iron between matte and slag indicate its tendency to be deported in the slag with increasing matte grade. The obtained distribution coefficients of sulfur between copper matte and slag from the experimental series of this study displayed the opposite trend along with increasing matte grade, as indicated in Figure 8(c). Similar increasing trends were also observed in the literature.[19] The results between matte and pure iron-silicate slag fit well with the observations by Sukhomlinov et al.[19]; however, the results between matte and alumina- and lime-containing slags are on the higher side of the previously reported results,[19] due to the differences of the alumina and lime concentrations in the slag mixtures.
Conclusions
The phase equilibrium of copper matte with three different silica-saturated slags (FeOx-SiO2, FeOx-SiO2-Al2O3, and FeOx-SiO2-Al2O3-CaO) was studied at 1300 °C and \( P_{{{\text{SO}}_{ 2} }} \) = 0.1 atm using a high-temperature equilibration technique followed by rapid quenching. EPMA phase analysis was used for obtaining phase compositions of the equilibrated assemblies directly from polished sections, which enabled the concentrations of the chemically dissolved species in each phase to be determined accurately. The technique eliminated potential uncertainties and errors in the phase assays due to entrainment of another phase. The experimentally measured results of the present study provide reliable fundamental thermodynamic data for improving resource efficiency in the pyrometallurgical recycling process of WEEE and for modeling the thermodynamic properties of iron silicate slags with alumina and lime modifiers.
The copper concentration in slag increased with an increasing matte grade, i.e., the prevailing oxygen partial pressure, whereas the iron and sulfur in the matte displayed the opposite trend. The addition of alumina and lime improved the copper concentration in the matte but had little effect on the sulfur and iron concentration in the matte. Copper concentration in pure iron-silicate slags increased with an increasing matte grade from 58 to 73 wt pct Cu. However, the amphoteric and basic oxide addition of alumina and lime effectively decreased the copper concentration to as low as 0.4 wt pct. Evidently, copper and sulfur tend toward distribution in the sulfide matte. In general, lower matte grades and addition of basic oxide favored copper deportment to matte.
References
[1]I. V. Kojo, A. Jokilaakso, and P. Hanniala: JOM, 2000, vol. 52 (2), pp. 57-61.
[2]T. Ahokainen and A. Jokilaakso: Can. Metall. Q., 1998, vol. 37 (3-4), pp. 275-83.
[3]A. Lennartsson, F. Engström, C. Samuelsson, B. Björkman, and J. Pettersson: J. Sustain. Metall., 2018, vol. 4 (2), pp. 222-32.
[4]M. A. H. Shuva, M. A. Rhamdhani, G. A. Brooks, S. Masood, and M. A. Reuter: J. Cleaner Prod., 2016, vol. 131, pp. 795-809.
U. Kuxmann and F. Y. Bor: World Metall.-Erzmet., 1965, vol. 18, pp. 441–50.
[6]F. J. Tavera and W. G. Davenport: Metall. Trans. B, 1979, vol. 10B, pp. 237-41.
[7]H. Li and W. J. Rankin: Metall. Mater. Trans. B, 1994, vol. 25B (1), pp. 79-89.
[8]M. Nagamori: Metall. Trans., 1974, vol. 5, pp. 531-38.
[9]R. Shimpo, S. Goto, O. Ogawa, and I. Asakura: Can. Metall. Q., 1986, vol. 25 (2), 113-21.
Z. Sun, T. Hidayat, P. C. Hayes, and E. Jak: Liquidus temperatures, major and minor elements equilibrium partitioning in copper smelting slag/matte/gas systems. In 8th International Copper Conference, Chile, 2013.
[11]H. Abdeyazdan, A. Fallah-Mehrjardi, T. Hidayat, M. Shevchenko, P. C. Hayes and E. Jak: J. Phase Equilib. Diffus., 2020, vol. 41, pp. 44-55.
[12]A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2017, vol. 48B (6), pp. 3002-16.
[13]A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2017, vol. 48B (6), pp. 3017-26.
[14]A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Int. J. Mater. Res., 2019, vol. 110B (6), pp. 489-95.
[15]A. Fallah-Mehrjardi, T. Hidayat, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2018, vol. 49B (4), pp. 1732-39.
[16]A. Fallah-Mehrjardi, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2018, vol. 49B (2), pp. 602-09.
[17]K. Avarmaa, H. Johto, and P. Taskinen: Metall. Mater. Trans. B, 2016, vol. 47B (1), pp. 244-55.
[18]K. Avarmaa, H. O’Brien, H. Johto, and P. Taskinen: J. Sustain. Metall., 2015, vol. 1 (3), pp. 216-28.
[19]D. Sukhomlinov, L. Klemettinen, H. O’Brien, P. Taskinen, and A. Jokilaakso: Metall. Mater. Trans. B, 2019, vol. 50B (6), pp. 2723-32.
[20]D. Shishin, T. Hidayat, A. Fallah-Mehrjardi, P. C. Hayes, S. A. Decterov, and E. Jak: J. Phase Equilib. Diffus., 2019, vol. 40, pp. 445-61.
M. Chen, K. Avarmaa, L. Klemettinen, H. O’Brien, D. Sukhomlinov, J. Shi, P. Taskinen, and A. Jokilaakso: Recovery of precious metals (Au, Ag, Pt, and Pd) from urban mining through copper smelting. Metall. Mater. Trans. B, 2020. https://doi.org/10.1007/s11663-020-01861-5.
[22]J. Gisby, P. Taskinen, J. Pihlasalo, Z. Li, M. Tyrer, J. Pearce, K. Avarmaa, P. Björklund, H. Davies, M. Korpi, S. Martin, L. Pesonen, and J. Robinson: Metall. Mater. Trans. B, 2017, vol. 48B (1), pp. 91-98.
SGTE Database for Pure Substances, Scientific Group Thermodata Europe. http://www.sgte.org/.
J. L. Pouchou and F. Pichoir: In 11th International Congress on X-Ray Optics and Microanalysis (ICXOM), J.D. Brown and R.H. Packwood, eds., Wiley, Ontario, Canada, 1986, pp. 249–56.
[25]A. Yazawa: Can. Metall. Q., 1974, vol. 13(3), 443-53.
Ž. Živković, N. Mitevska, I. Mihajlović, and Đ. Nikolić: J. Min. Metall. Sect. B, 2009, vol. 45, pp. 23-34.
[27]H. G. Kim and H. Y. Sohn: Metall. Mater. Trans. B, 1998, vol. 29B (3), pp. 583-90.
[28]P. J. Mackey: Can. Metall. Q., 1982, vol. 21 (3), pp. 221-60.
B. Keyworth: in Proc. Precious Metals 1982, California, Pergamon, 1983, pp. 509–37. https://doi.org/10.1016/B978-0-08-025396-1.50044-0.
J. F. Elliott, M. Gleiser, and V. Ramakrishna: Thermochemistry for Steelmaking, Addison-Wesley, Reading, MA, 1963, pp. 678-79
[31]C. Wagner: Metall. Trans. B, 1975, vol. 6B (3), pp. 405-09.
J. A. Duffy, M. D. Ingram, and I. D. Sommerville: J. Chem. Soc Faraday Trans. I, 1978, vol. 14, pp. 1410-19.
[33]T. Hidayat, A. Fallah-Mehrjardi, P. C. Hayes, and E. Jak: Metall. Mater. Trans. B, 2018, vol. 49B (4), 1750-65.
K. Yamaguchi: in Proc. Extraction 2018, Ottawa, Springer, Cham, 2018, pp. 797–804. https://doi.org/10.1007/978-3-319-95022-8_63
G. Roghani, M. Hino, and K. Itagaki: in Proc. 5th Int. Conf. on Molten Slags, Fluxes and Salts. Iron & Steel Society, Warrendale, PA, 1997, pp. 693–703.
G. Roghani, M. Hino, and K. Itagaki: Mater. Trans., JIM, 1997, vol. 38 (8), pp. 707–13.
Y. Takeda: in Proc. ’97 Conf. Molten Slags, Fluxes and Salts, Sydney, Iron & Steel Society, Iron & Steel Society, Warrendale, PA, pp. 329–39.
[38]G. Roghani, Y. Takeda, and K. Itagaki: Metall. Mater. Trans. B, 2000, vol. 31B (4), pp. 705-12.
[39]H. Jalkanen: Scand. J. Metall., 1981, vol. 10 (4), pp. 177-84.
[40]M. Nagamori and P. J. Mackey: Metall. Trans. B, 1978, vol. 9B (2), pp. 255-65.
G. Roghani, J. C. Font, M. Hino, and K. Itagaki: Mater. Trans. JIM, 1996, 37 (10), pp. 1574–79.
J. M. Font, Y. Takeda, and K. Itagaki: Mater. Trans. JIM, 1998, vol. 39 (6), pp. 652-57.
[43]G. H. Kaiura, K. Watanabe, and A. Yazawa: Can. Metall. Q., 1980, vol. 19 (2), pp. 191-200.
C.M. Acuna and A. Yazawa: JOM, 1997, vol. 49 (10).
[45]E. T. Turkdogan: Physicochemical properties of molten slags and glasses, London: The Metals Society, 1983, pp. 99-122.
Acknowledgments
Open access funding provided by Aalto University. This work was partly financed by the Aalto University School of Chemical Engineering and the SYMMET project financed by Business Finland (Grant 3891/31/2018), and it utilized the Academy of Finland’s RawMatTERS Finland Infrastructure (RAMI) based at Aalto University, GTK Espoo, and VTT Espoo. The contribution of Mr. Lassi Pakkanen of the Geological Survey of Finland (GTK) by conducting the EPMA analyses is especially appreciated. Min Chen gratefully acknowledges the support from a scholarship from the China Scholarship Council (Grant Number 201806370217).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Manuscript submitted March 2, 2020.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, M., Avarmaa, K., Klemettinen, L. et al. Experimental Study on the Phase Equilibrium of Copper Matte and Silica-Saturated FeOx-SiO2-Based Slags in Pyrometallurgical WEEE Processing. Metall Mater Trans B 51, 1552–1563 (2020). https://doi.org/10.1007/s11663-020-01874-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11663-020-01874-0