Abstract
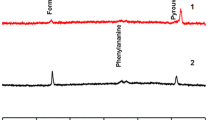
A metabolomic classification of commercial and unprocessed organic honeys from Zambia and Botswana based on geographic and floral origins was done forthwith. Classification using gas chromatography–mass spectrometry/solid phase microextraction (GC–MS/SPME) was accomplished on three commercial and three unprocessed organic honeys. The Automated Mass spectral Deconvolution and Identification System (AMDIS), Metab R, an R platform application and MINITAB version 14 partial least squares (PLS) were used for data processing. Sixteen volatile metabolites in three commercial and 40 in three unprocessed organic honeys were identified, confirmed and formed the basis for differentiation. Database search showed that the honeys were polyfloral with major ingredients coming from common flowering plants, conifers and other gymnosperms such as Carica papaya L. (papaya), Monstera deliciosa (ceriman) and fruits, i.e., guava, melon and pineapple, endemic in the areas from which the honeys originated from.





Similar content being viewed by others
References
UNFAO (2017) https://www.fao.org/3/y4351e/y4351e08.htm. Accessed 18 Feb 2020
Jandri J, Frew RD, Fernandez-Cedi LN, Cannavan A (2017) An investigative study on discrimination of honey of various floral and geographical origins using UPLC–QToF MS and multivariate data analysis. Food Control 72:189–197
Piazza MG, Persano Persano Oddo L (2004) Bibliographical review of the main European unifloral honeys. Apidologie 35:S94–S111
Ribeiro ROR, Mársico ET, Carneiro C, Monteiro MLG, Conte Júnior CA, Mano S, de Jesus EFO (2014) Classification of Brazilian honeys by physical and chemical analytical methods and low field nuclear magnetic resonance (LF 1H NMR). LWT Food Sci Technol 55:90–95
Chen L, Wang J, Ye Z, Zhao J, Xue X, Heyden YV, Sun Q (2012) Classification of Chinese honeys according to their floral origin by near infrared spectroscopy. Food Chem 135:338–342
Fechner DC, Moresi A, RuizDíaz JD, Pellerano RG, Vazquez FA (2016) Multivariate classification of honeys from Corrientes (Argentina) according to geographical origin based on physicochemical properties. Food Biosci 15:49–54
Chakir A, Romane A, Marcazzan GL, Ferrazzi P (2016) Physicochemical properties of some honeys produced from different plants in Morocco. Arab J Chem 9:S946–S954
Spiteri M, Rogers KM, Jamin E, Thomas F, Guyader S, Lees M, Rutledge DN (2017) Combination of 1H NMR and chemometrics to discriminate manuka honey from other floral honey types from Oceania. Food Chem 217:766–772
Manzanares AB, García ZH, Galdón BR, Rodríguez-Rodríguez EM, Romero CD (2017) Physicochemical characteristics and pollen spectrum of monofloral honeys from Tenerife, Spain. Food Chem 228:441–446
Kortesniemi M, Slupsky CM, Ollikka T, Kauko L, Spevacek AR, Sjovall O, Yang B, Kallio H (2014) Sensory and chemical profiles of Finnish honeys of different botanical origins and consumer preferences. Food Chem 246:351–359
Boussaid A, Chouaibi M, Rezig L, Hellal R, Donsì F, Ferrari G, Hamdi S (2018) Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab J Chem 11:265–274
Moniruzzaman M, Rodriguez I, Rodriguez-Cabo T, Cela R, Sulaiman SA, Gan SH (2014) Assessment of dispersive liquid–liquid microextraction conditions for gas chromatography time-of-flight mass spectrometry identification of organic compounds in honey. J Chromatogr A 1368:26–36
Seisonen S, Kivima E, Vene K (2015) Characterisation of the aroma profiles of different honeys and corresponding flowers using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem 169:34–40
Karabagias IK, Badeka A, Kontakos S (2014) Characterization and classification of Thymus capitatus (L.) honey according to geographical origin based on volatile compounds, physicochemical parameters and chemometrics. Food Res Int 55:363–372
R Core Team (2018) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 18 Feb 2020
Aggio R, Villas-Boas SG, Ruggiero K (2011) Metab: an R package for high-throughput analysis of Metabolomics data generated by GC–MS. Bioinform J 16:2316–2318
D’Arcy D, Mallard WG (2004) AMDIS—user guide. Department of Commerce Technology Administration, National Institute of Standards and Technology (NIST). https://www.nist.gov/sites/default/files/documents/srd/AMDISMan.pdf. Accessed 23 Aug 2019
Piasenzotto L, Gracco L, Conte L (2003) Solid phase microextraction (SPME) applied to honey quality control. J Sci Food Agric 83:1037–1044
Špánik I, Pazˇitná A, Šiška P, Szolcsányi P (2014) The determination of botanical origin of honeys based on enantiomer distribution of chiral volatile organic compounds. Food Chem 158:497–503
Verzera A, Campisi S, Zappala M, Bonaccorsi I (2001) SPME-GC–MS analysis of honey volatile components for the characterization of different floral origin. Am Lab 33(15):18–21
Ravikkumar VR, Gopal V, Ravichandran N, Brindha P, Sudha T (2014) Analysis of phytochemical constituents of stem bark extracts of Zanthoxylum tetraspermum Wight & Arn. Res J Pharm Biol Chem Sci 3(4):391–402
Yadav SA, Ramalingam S, Jebamalairaj A, Subban R, Sundaram KM (2016) Biochemical fingerprint and pharmacological applications of Barleria noctiflora L.f. leaves. J Complement Integr Med 13(4):365–376
Ishola FT, Aboaba SA, Choudhary MI, Ekundayo O (2017) Chemical and biological assessments of the essential oils of Chrysophyllum albidum G. Don J Agric Sci Technol A 7:234–245
Belsito D, Bickers D, Bruze M, Calow P, Greim H, Hanifin JM, Rogers AE, Saurat JH, Sipes IG, Tagami H (2011) A toxicologic and dermatologic assessment of cyclic and non-cyclic terpene alcohols when used as fragrance ingredients. Food Chem Toxicol 49(S2):S174–S182
Hashizume M, Gordon MH, Mottram DS (2007) Light-induced off-flavor development in cloudy apple juice. J Agric Food Chem 55(22):9177–9182
Patil SS, Magdum CS (2013) Synthesis and study some derivatives of pyrimidine for their antibacterial activity. Int J Univ Pharm Biol Sci 2(3):469–476
Ponnusamy SS, Banu S, Vedigounder M, Narayanswamy D (2018) GC MS/MS analysis of bioactive compounds in alcoholic seed extract of Gauzuma ulmifolia Lam. Pharmacogn J 10(1):194–197
Sathyaprabha G, Senthil R (2017) Molecular characterization and phytochemical studies of Pleurotus sp. to unveil species diversity and pharmacological activities. The Free Library. 2017 Oriental Scientific Publishing Company 03 Mar. 2020. https://www.thefreelibrary.com/Molecular+characterization+and+phytochemical+studies+of+Pleurotus+sp...-a0500683453
Sayumi Yamazoe S, Koji Hasegawa K, Hideyuki Shigemori H (2006) Structure–activity relationship of acetylenes from galls of Hedera rhombeaas plant growth inhibitors. Z Naturforsch 61c:536–540
Flament I (2001) Coffee flavor chemistry. Wiley, Chichester
Pereira J, Pereira J, Câmara JS (2011) Effectiveness of different solid-phase microextraction fibres for differentiation of selected Madeira island fruits based on their volatile Metaboliteprofile—identification of novel compounds. Talanta 83:899–906
Prenafeta-Boldú FX, Luykx DMAM, Vervoort J, de Bont JAM (2001) Fungal Metabolism of toluene: monitoring of fluorinated analogs by 19F nuclear magnetic resonance spectroscopy. Appl Environ Microbiol 56:1347–1351
Mazida MM, Salleh MM, Hassan O (2005) Analysis of volatile aroma compounds of fresh chilli (Capsicum annuum) during stages of maturity using solid phase microextraction (SPME). J Food Comp Anal 18(5):427–437
Teerawanichpan P, Qiu X (2010) Fatty acyl–CoA reductase and wax synthase from Euglena gracilis in the biosynthesis of medium-chain wax esters. Lipids 45:263–273
Usha PR, Naidu MU (2004) Randomised, double-blind, parallel, placebo-controlled study of oral glucosamine, methylsulfonylmethane and their combination in osteoarthritis. Clin Drug Investig 24(6):353–363
Acknowledgements
The authors thank the University of Botswana for material support and the Ministry of Agriculture in Botswana; for providing information on beekeepers and granting the research permit to do the project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sichilongo, K., Padiso, T. & Turner, Q. AMDIS-Metab R data manipulation for the geographical and floral differentiation of selected honeys from Zambia and Botswana based on volatile chemical compositions using SPME–GC–MS. Eur Food Res Technol 246, 1679–1690 (2020). https://doi.org/10.1007/s00217-020-03523-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03523-x