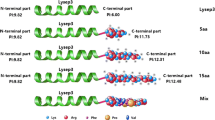
Abstract
Developing chimeric lysins with a wide lytic spectrum is important in fighting against Gram-negative pathogenic bacteria. In the present work, a novel chimerical lysin, LytAmfi, was constructed by fusing the bacteriophage endolysin Lyt μ1/6 with an amphipathic cationic peptide derived from T4 lysozyme. The result showed that the LytAmfi had not only lytic activity similar to parental Streptomyces aureofaciens endolysin Lyt μ1/6 but also broadened lytic activity against Gram-negative strains. Our work demonstrated that generating a novel chimeric lysin with an extended lytic spectrum was workable by fusing an active modular endolysin with an amphipathic cationic peptide that could penetrate the outer membrane of Gram-negative bacteria including Escherichia coli, Pseudomonas agglomerans, Acinetobacter lwoffii, Hafnia alvei, Citrobacter freundii and enable access of the lysin to lyse the bacteria ‘from within’ the host cell without pre-treatment of its outer membrane. Therefore, lysins with an extended spectrum of lytic activity would be of appreciable therapeutic value.






Similar content being viewed by others
Abbreviations
- CBD:
-
C-terminal cell-wall binding domain
- CD-search:
-
Conserved domain Search service
- CFU:
-
Colony-forming unit
- ECD:
-
SN-terminal catalytic domain
- FPLC:
-
Fast protein liquid chromatography
- GFP:
-
Green fluorescent protein
- HEPES:
-
2-[4-(2-hydroxyethyl)piperazin-1-yl]ethane sulfonic acid
- IPTG:
-
Isopropyl β-D-1-thiogalactopyranoside
- LB:
-
Lysogeny broth
- LBS:
-
Lysogeny broth with high salt
- NCBI:
-
National Center for Biotechnology Information
- Ni-NTA:
-
Nickel-nitrilotriacetic acid
- OD:
-
Optical density
- PCR:
-
Polymerase chain reaction
- PDB:
-
Protein data bank
- PGRP:
-
Peptidoglycan recognition proteins
- PHAGE:
-
Bacteriophage
- PSI-BLAST:
-
Position-specific iterated basic local alignment search tool
- SDS-PAGE:
-
Sodium dodecyl sulphate polyacrylamide gel electrophoresis
- TB:
-
Terrific broth
- TBG:
-
Terrific broth with glycylglycine
References
Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman DJ (1998) Gapped blast and psi-blast: a new generation of protein database search programs. FASEB J 12:A1325. https://doi.org/10.1093/nar/25.17.3389
Bagby S, Tong KI, Liu D, Alattia J, Ikura M (1997) The button test: a small scale method using microdialysis cells for assessing protein solubility at concentrations suitable for NMR. J Biomol NMR 10:279–282. https://doi.org/10.1023/A:1018359305544
Bhandari P, Gowrishankar J (1997) An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. J Bacteriol 179:4403–4406. https://doi.org/10.1128/jb.179.13.4403-4406.1997
Bondos SE, Bicknell A (2003) Detection and prevention of protein aggregation before, during, and after purification. Anal Biochem 316:223–231. https://doi.org/10.1016/S0003-2697(03)00059-9
Briers Y, Walmagh M, Grymonprez B, Biebl M, Pirnay JV, Defraine V, Michiels J, Cenens W, Aertsen A, Miller S, Lavigne R (2014a) Art-175 is a highly efficient antibacterial against multidrug-resistant strains and persisters of Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:3774–3784. https://doi.org/10.1128/AAC.02668-14
Briers Y, Walmagh M, Van Puyenbroeck V, Cornelissen A, Cenens W, Aertsen A, Oliveira H, Azeredo J, Verween G, Pirnay JP, Miller S, Volckaert G, Lavigne R (2014b) Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. mBio 5:e01379–14. https://doi.org/10.1128/mBio.01379-14
Burley SK, Berman HM, Bhikadiya C, Bi C, Chen L, Di Costanzo L, Christie C, Dalenberg K, Duarte JM, Dutta S, Feng Z, Ghosh S, Goodsell DS, Green RK, Guranovic V, Guzenko D, Hudson BP, Kalro T, Liang Y, Lowe R, Namkoong H, Peisach E, Periskova I, Prlic A, Randle C, Rose A, Rose P, Sala R, Sekharan M, Shao C, Tan L, Tao YP, Valasatava Y, Voigt M, Westbrook J, Woo J, Yang H, Young J, Zhuravleva M, Zardecki C (2019) RCSB protein data bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res 47:D464–D474. https://doi.org/10.1093/nar/gky1004
Bitencourt-Ferreira G, de Azevedo WF Jr (2019) Molegro virtual Docker for docking. Methods Mol Biol 2053:149–167. https://doi.org/10.1007/978-1-4939-9752-7_10
Büyükcam A, Tuncer Ö, Gür D, Sancak B, Ceyhan M, Cengiz AB, Kara A (2018) Clinical and microbiological characteristics of Pantoea agglomerans infection in children. J Infect Public Health 11:304–309. https://doi.org/10.1016/j.jiph.2017.07.020
Bystroff C, Krogh A (2008) Hidden Markov models for prediction of protein features. Methods Mol Biol 413:173–198. https://doi.org/10.1007/978-1-59745-574-9_7
Cheng X, Zhang X, Pflugrath JW, Studier FW (1994) The structure of bacteriophage T7 lysozyme, a zinc amidase and an inhibitor of T7 RNA polymerase. Proc Natl Acad Sci U S A 91:4034–4038. https://doi.org/10.1073/pnas.91.9.4034
Chotár M, Vidová B, Godány A (2006) Development of specific and rapid detection of bacterial pathogens in dairy products by PCR. Folia Microbiol 51:639–646. https://doi.org/10.1007/bf02931632
Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2016) GenBank. Nucleic Acids Res 44:D67–D72. https://doi.org/10.1093/nar/gkv1276
Cowell JL (1974) Energetics of glycylglycine transport in Escherichia coli. J Bacteriol 120:139–146
Crooks GE, Hon G, Chandonia J, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14:1188–1190. https://doi.org/10.1101/gr.849004
Dutkiewicz J, Mackiewicz B, Kinga Lemieszek M, Golec M, Milanowski J (2016) Pantoea agglomerans: a mysterious bacterium of evil and good. Part III. Deleterious effects: infections of humans, animals and plants. Ann Agric Environ Med 23:197–205. https://doi.org/10.5604/12321966.1203878
Düring K, Porsch P, Mahn A, Brinkmann O, Gieffers W (1999) The non-enzymatic microbicidal activity of lysozymes. FEBS Lett 449:93–100. https://doi.org/10.1016/S0014-5793(99)00405-6
Dziarski R (2004) Peptidoglycan recognition proteins (PGRPs). Mol Immunol 40:877–886. https://doi.org/10.1186/gb-2006-7-8-232
Farkašovská J, Godány A, Vlček Č (2003) Identification and characterization of an endolysin encoded by the Streptomyces aureofaciens phage μ1/6. Folia Microbiol 48:737–744. https://doi.org/10.1007/bf02931507
Farkašovská J, Godány A (2008) The lysis system of the Streptomyces aureofaciens phage μ1/6. Curr Microbiol 57:631–637. https://doi.org/10.1007/s00284-008-9255-0
Farkašovská J, Godány A (2016) Characterization of the N-terminal catalytic domain of Lyt μ1/6, an Endolysin from Streptomyces aureofaciens phage μ1/6. Folia Microbiol 73:602–610. https://doi.org/10.1007/s00284-016-1100-2
Fenton M, Ross P, Mcauliffe O, O'Mahony J, Coffey A (2010) Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:9–16. https://doi.org/10.4161/bbug.1.1.9818
Ferrer M, Chernikova TN, Yakimov MM, Golyshin PN, Timmis KN (2003) Chaperonins govern growth of Escherichia coli at low temperatures. Nat Biotechnol 21:1266–1267. https://doi.org/10.1038/nbt1103-1266
Fischetti VA (2006) Using phage lytic enzymes to control pathogenic bacteria. BMC Oral Health 6:S1–S16. https://doi.org/10.1186/1472-6831-6-S1-S16
Hartl FU, Hayer-Hartl M (2002) Protein folding. Molecular chaperones in the cytosol: from nascent chain to folded protein. Science 295:1852–1858. https://doi.org/10.1126/science.1068408
Jayol A, Saly M, Nordmann P, Ménard A, Poirel L, Dubois V (2017) Hafnia, an enterobacterial genus naturally resistant to colistin revealed by three susceptibility testing methods. J Antimicrob Chemother 72:2507–2511. https://doi.org/10.1093/jac/dkx154
Kaper T, Brouns SJJ, Geerling ACM, De Vos WM, Van der Oost J (2002) DNA family shuffling of hyperthermostable β-glycosidases. Biochem J 368:461–470. https://doi.org/10.1042/BJ20020726
Laird T (1997) Ullmann’s encyclopedia of industrial chemistry. Org. Process Res Dev 5:391–392. https://doi.org/10.1021/op970020u
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Lepre CA, Moore JM (1998) Microdrop screening: a rapid method to optimize solvent conditions for NMR spectroscopy of proteins. J Biomol NMR 12:493–499. https://doi.org/10.1023/A:1008353000679
Liu C, Xu Z, Gupta D, Dziarski R (2001) Peptidoglycan recognition proteins: a novel family of four human innate immunity pattern recognition molecules. J Biol Chem 276:34686–34694. https://doi.org/10.1074/jbc.M105566200
Liu LH, Wang NY, Wu AY, Lin CC, Lee CM, Liu CP (2018) Citrobacter freundii bacteremia: risk factors of mortality and prevalence of resistance genes. J Microbiol Immunol Infect 51:565–572. https://doi.org/10.1016/j.jmii.2016.08.016
Loessner MJ, Kramer K, Ebel F, Scherer S (2002) C-terminal domains of listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol Microbiol 44:335–349. https://doi.org/10.1046/j.1365-2958.2002.02889.x
Loessner MJ (2005) Bacteriophage endolysins – current state of research and applications. Curr Opin Microbiol 8:480–487. https://doi.org/10.1016/j.mib.2005.06.002
Lu S, Wang J, Chitsaz F, Derbyshire MK, Geer RC, Gonzales NR, Gwadz M, Hurwitz DI, Marchler GH, Song JS, Thanki N, Yamashita RA, Yang M, Zhang D, Zheng C, Lanczycki CJ, Marchler-Bauer A (2020) CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res 48:D265–D268. https://doi.org/10.1093/nar/gkz991
Lukáčik P, Barnard TJ, Keller PW, Chaturvedi KS, Seddiki N, Fairman JW, Noinaj N, Kirby TL, Henderson JP, Steven AC, Hinnebusch BJ, Buchanan SK (2012) Structural engineering of a phage lysin that targets gram-negative pathogens. Proc Natl Acad Sci U S A 109:9857–9862. https://doi.org/10.1073/pnas.1203472109
Mainardi JL, Mugnier P, Coutrot A, Buu-Hoï A, Collatz E, Gutmann L (1997) Carbapenem resistance in a clinical isolate of Citrobacter freundii. Antimicrob Agents Chemother 41:2352–2354. https://doi.org/10.1128/AAC.41.11.2352
Mellroth P, Karlsson J, Steiner H (2003) A scavenger function for a drosophila peptidoglycan recognition protein. J Biol Chem 278:7059–7064. https://doi.org/10.1074/jbc.M208900200
Michel T, Relchhart J, Hoffmann JA, Royet J (2001) Drosophila toll is activated by gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature 414:756–759. https://doi.org/10.1038/414756a
Mittal S, Sharma M, Yadav A, Bala K, Chaudhary U (2015) Acinetobacter lwoffii an emerging pathogen in neonatal ICU. Infect Disord Drug Targets 15:184–188. https://doi.org/10.2174/1871526515666150826114745
Mogk A, Mayer MP, Deuerling E (2002) Mechanisms of protein folding: molecular chaperones and their application in biotechnology. ChemBioChem 3:807–814. https://doi.org/10.1002/1439-7633(20020902)3:9<807:AID-CBIC807>3.0.CO;2-A
Oliveira H, Azeredo J, Lavigne R, Kluskens LD (2012) Bacteriophage endolysins as a response to emerging foodborne pathogens. Trends Food Sci Technol 28:103–115. https://doi.org/10.1016/j.tifs.2012.06.016
Ou W, Park Y, Zhou H (2002) Effect of osmolytes as folding aids on creatine kinase refolding pathway. Int J Biochem Cell Biol 34:136–147. https://doi.org/10.1016/S1357-2725(01)00113-3
PDB ID: 1yB0
Low LY, Yang C, Perego M, Osterman A, Liddington RC (2005) Structure and lytic activity of a bacillus anthracis prophage endolysin. J Biol Chem 280:35433–35439. https://doi.org/10.1074/jbc.M502723200
PDB ID: 3D2Y
Kerff F, Petrella S, Mercier F, Sauvage E, Herman R, Pennartz A, Zervosen A, Luxen A, Frère JM, Joris B, Charlier P (2010) Specific structural features of the N-acetylmuramoyl-l-alanine amidase AmiD from Escherichia coli and mechanistic implications for enzymes of this family. J Mol Biol 397:249–259. https://doi.org/10.1016/j.jmb.2009.12.038
PDB ID: 3rdR
Low LY, Yang C, Perego M, Osterman A, Liddington R (2011) Role of net charge on catalytic domain and influence of cell wall binding domain on bactericidal activity, specificity, and host range of phage lysins. J Biol Chem 286:34391–34403. https://doi.org/10.1074/jbc.M111.244160
Rodriguez-Brito B, Li L, Wegley L, Furlan M, Angly BFM, Buchanan J, Desnues C, Dinsdale E, Edwards R, Felts B, Haynes M, Liu H, Lipson D, Mahaffy J, Martin-Cuadrado AB, Mira A, Nulton J, Pašić L, Rayhawk S, Rodriguez-Mueller J, Rodriguez-Valera F, Salamon P, Srinagesh S, Thingstad TF, Tran T, Thurber RV, Willner D, Youle M, Rohwer F (2010) Viral and microbial community dynamics in four aquatic environments. ISME J 4:739–751. https://doi.org/10.1038/ismej.2010.1
Rost B (1996) PHD: predicting one-dimensional protein structure by profile-based neural networks. Methods Enzymol 266:525–539. https://doi.org/10.1016/s0076-6879(96)66033-9
Sambrook JF, Russell D (2001) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sawyer JR, Schlom J, Kashmiri SVS (1994) The effects of induction conditions on production of a soluble anti-tumor sFv in Escherichia coli. Protein Eng 7:1401–1406. https://doi.org/10.1093/protein/7.11.1401
Sen TZ, Jernigan RL, Garnier J, Kloczkowski A (2005) GOR V server for protein secondary structure prediction. Bioinformatics 21:2787–2788. https://doi.org/10.1093/bioinformatics/bti408
Schein CH (1989) Production of soluble recombinant proteins in bacteria. Nat Biotechnol 7:1141–1149. https://doi.org/10.1186/2193-1801-2-89
Schmelcher M, Donovan DM, Loessner MJ (2012) Bacteriophage endolysins as novel antimicrobials. Future Microbiol 7:1147–1171. https://doi.org/10.2217/fmb.12.97
Schwarz E, Lilie H, Rudolph R (1996) The effect of molecular chaperones on in vivo and in vitro folding processes. Biol Chem 377:411–416. https://doi.org/10.1515/bchm3.1996.377.7-8.411
Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K et al (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using clustal omega. Mol Syst Biol 7:539. https://doi.org/10.1038/msb.2011.75
Sørensen HP, Mortensen KK (2005) Soluble expression of recombinant proteins in the cytoplasm of Escherichia coli. Microb Cell Factories 4:1. https://doi.org/10.1186/1475-2859-4-1
The UniProt Consortium (2019) UniProt: a worldwide hub of protein knowledge. Nucleic Acids Res 47:D506–D515. https://doi.org/10.1093/nar/gky1049
Tišáková L, Vidová B, Farkašovská J, Godány A (2014) Bacteriophage endolysin Lyt μ1/6: characterization of the C-terminal binding domain. FEMS Microbiol Lett 350:199–208. https://doi.org/10.1111/1574-6968.12338
Viard T, Cossard R, Duguet M, Bouthier De La Tour C (2004) Thermotoga maritima-Escherichia coli chimeric topoisomerases: answers about involvement of the carboxyl-terminal domain in DNA topoisomerase. J Biol Chem 279:30073–30080. https://doi.org/10.1074/jbc.M309692200
Vidová B, Šramková Z, Tišáková L, Oravkinová M, Godány A (2014) Bioinformatics analysis of bacteriophage and prophage endolysin domains. Biologia 69:541–556. https://doi.org/10.2478/s11756-014-0358-8
Wang ZM, Li X, Cocklin RR, Wang M, Wang M, Fukase K, Inamura S, Kusumoto S, Gupta D, Dziarski R (2003) Human peptidoglycan recognition protein-L is an N-acetylmuramoyl-L-alanine amidase. J Biol Chem 278:49044–49052. https://doi.org/10.1074/jbc.M307758200
Wanger A, Chavez V, Huang RSP, Wahed A, Actor JK, Dasgupta A (2017) Microbiology and molecular diagnosis in pathology. Elsevier Inc, Amsterdam
Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D (2000) A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci U S A 97:13772–13777. https://doi.org/10.1073/pnas.97.25.13772
Yang H, Zhang Y, Yu J, Huang Y, Zhang X, Wei H (2014) Novel chimeric lysin with high-level antimicrobial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo. Antimicrob Agents Chemother 58:536–542. https://doi.org/10.1128/AAC.01793-13
Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y (2015) The I-TASSER suite: protein structure and function prediction. Nat Methods 12:7–8. https://doi.org/10.1038/nmeth.3213
Acknowledgments
We would like to thank especially Dr. Lubica Urbanikova for valuable recommendations, proposal and help in predicting the tertiary structure of Lyt μ1/6. The authors also acknowledge doc. Dr. Peter Pristas and the Institute of Biology and Ecology, Pavol Jozef Safarik University in Kosice for providing of Gram-negative bacterial isolates. This work was financially supported by APVV grant APVV-16-0173 and VEGA grant no.2/0123/14 from the Scientific Grant Agency of the Ministry of Education, Sciences, Research and Sports of the Slovak Republic, and Slovak Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The authors declare no ethical considerations to apply.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mancoš, M., Šramková, Z., Peterková, D. et al. Functional expression and purification of tailor-made chimeric endolysin with the broad antibacterial spectrum. Biologia 75, 2031–2043 (2020). https://doi.org/10.2478/s11756-020-00508-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11756-020-00508-9