2D-Cross Correlation Spectroscopy Coupled with Molecular Fluorescence Spectroscopy for Analysis of Molecular Structure Modification of Camel Milk and Cow Milk Mixtures during Coagulation
Abstract
:1. Introduction
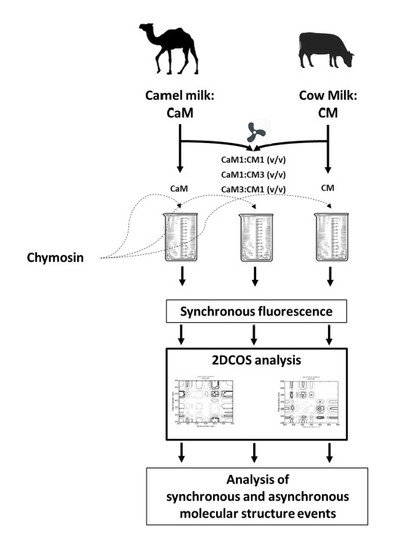
2. Materials and Methods
2.1. Materials
2.2. Fluorescence Spectroscopy
2.3. Two-Dimensional Correlation Spectroscopy
3. Results and Discussion
3.1. Spectral Analysis and Comparison between Milk Formulations
3.2. Analysis of 2D COS Synchronous and Asynchronous Spectra
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Faye, B.; Konuspayeva, G. The sustainability challenge to the dairy sector e The growing importance of non-cattle milk production worldwide. Int. Dairy J. 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Farah, Z.; Rettenmaier, R.; Atkins, D. Vitamin content of camel milk. Int. J. Vitam. Nutr. Res. 1992, 62, 30–33. [Google Scholar] [PubMed]
- Sawaya, W.N.; Khalil, J.K. Chemical Composition and Nutritional Quality of Camel Milk. J. Food Sci. 1984, 49, 744–747. [Google Scholar] [CrossRef]
- Khalesi, M.; Salami, M.; Moslehishad, M.; Winterburn, J.; Moosavi-Movahedi, A.A. Biomolecular content of camel milk: A traditional superfood towards future healthcare industry. Trends Food Sci. Technol. 2017, 62, 49–58. [Google Scholar] [CrossRef]
- Lobato-Calleros, C.; Vernon-Carter, E.J.; Hornelas-Uribe, Y. Microstructure and texture of cheese analogs containing different types of fat. J. Texture Stud. 1998, 29, 569–586. [Google Scholar] [CrossRef]
- Lamichhane, P.; Kelly, A.L.; Sheehan, J.J. Symposium review: Structure-function relationships in cheese. J. Dairy Sci. 2018, 101, 2692–2709. [Google Scholar] [CrossRef]
- Boubellouta, T.; Dufour, É. Effects of Mild Heating and Acidification on the Molecular Structure of Milk Components as Investigated by Synchronous Front-Face Fluorescence Spectroscopy Coupled with Parallel Factor Analysis. Appl. Spectrosc. 2008, 62, 490–496. [Google Scholar] [CrossRef]
- Dubayová, K.; Kušnír, J.; Makuchová, V. Synchronous fluorescence fingerprint as the identity card of food products. Chem. Pap. 1998, 52, 797. [Google Scholar]
- Hammami, M.; Rouissi, H.; Salah, N.; Selmi, H.; Al-Otaibi, M.; Blecker, C.; Karoui, R. Fluorescence spectroscopy coupled with factorial discriminant analysis technique to identify sheep milk from different feeding systems. Food Chem. 2010, 122, 1344–1350. [Google Scholar] [CrossRef]
- Boubellouta, T.; Galtier, V.; Dufour, É. Effects of added minerals (calcium, phosphate, and citrate) on the molecular structure of skim milk as investigated by mid-infrared and synchronous fluorescence spectroscopies coupled with chemometrics. Appl. Spectrosc. 2009, 63, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Boubellouta, T.; Galtier, V.; Dufour, É. Structural changes of milk components during acid-induced coagulation kinetics as studied by synchronous fluorescence and mid-infrared spectroscopy. Appl. Spectrosc. 2011, 65, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Loudiyi, M.; Aït-Kaddour, A. Evaluation of the effect of salts on chemical, structural, textural, sensory and heating properties of cheese: Contribution of conventional methods and spectral ones. Crit. Rev. Food Sci. Nutr. 2019, 59, 2442–2457. [Google Scholar] [CrossRef] [PubMed]
- Loudiyi, M.; Karoui, R.; Rutledge, D.N.; Lavigne, R.; Montel, M.; Aït-kaddour, A. Contribution of fluorescence spectroscopy and independent components analysis to the evaluation of NaCl and KCl effects on molecular-structure and fat melting temperatures of Cantal-type cheese. Int. Dairy J. 2017, 73, 116–127. [Google Scholar] [CrossRef]
- Loudiyi, M.; Karoui, R.; Rutledge, D.N. Fluorescence spectroscopy coupled with independent components analysis to monitor molecular changes during heating and cooling of Cantal-type cheeses with different NaCl and KCl contents. J. Sci. Food Agric. 2018, 98, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Noda, I. Two-dimensional infrared spectroscopy of synthetic and biopolymers. Bull. Am. Phys. Soc. 1986, 31, 520–524. [Google Scholar]
- Noda, I. Two-dimensional infrared (2D IR) spectroscopy: Theory and applications. Appl. Spectrosc. 1990, 44, 550–561. [Google Scholar] [CrossRef]
- Noda, I. Generalized two-dimensional correlation method applicable to infrared, Raman, and other types of spectroscopy. Appl. Spectrosc. 1993, 47, 1329–1336. [Google Scholar] [CrossRef]
- Ozaki, Y.; Noda, I. Potential of generalised two-dimensional correlation spectroscopy in the near infrared region. J. Near Infrared Spectrosc. 1996, 4, 85–99. [Google Scholar] [CrossRef]
- Wang, Y.; Murayama, K.; Myojo, Y.; Tsenkova, R.; Hayashi, N.; Ozaki, Y. Two-dimensional Fourier transform near-infrared spectroscopy study of heat denaturation of ovalbumin in aqueous solutions. J. Phys. Chem. B 1998, 102, 6655–6662. [Google Scholar] [CrossRef]
- Axelrod, D.; Koppel, D.E.; Schlessinger, J.; Elson, E.; Webb, W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976, 16, 1055. [Google Scholar] [CrossRef] [Green Version]
- Koppel, D.E.; Axelrod, D.; Schlessinger, J.; Elson, E.L.; Webb, W.W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys. J. 1976, 16, 1315–1329. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Chen, J.; Wu, X.; Zhou, Q.; Sun, S. Rapid discrimination of Panax notogeinseng of different grades by FT-IR and 2DCOS-IR. J. Mol. Struct. 2016, 1124, 131–137. [Google Scholar] [CrossRef]
- Chen, N.; Chen, N.; Li, J.; Cao, C.; Wang, J. Rapid authentication of different ages of tissue-cultured and wild Dendrobium huoshanense as well as wild Dendrobium henanense using FTIR and 2D-COS IR. J. Mol. Struct. 2015, 1101, 101–108. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Murphy, K.R.; Hambly, A.; Stuetz, R.M.; Khan, S.J. Fluorescence as a potential monitoring tool for recycled water systems : A review. Water Res. 2009, 43, 863–881. [Google Scholar] [CrossRef]
- Hur, J.; Jung, K.; Mee, Y. Characterization of spectral responses of humic substances upon UV irradiation using two-dimensional correlation spectroscopy. Water Res. 2011, 45, 2965–2974. [Google Scholar] [CrossRef]
- Hur, J.; Lee, B. Comparing the Heterogeneity of Copper-Binding Characteristics for Two Different-Sized Soil Humic Acid Fractions Using Fluorescence Quenching Combined with 2D-COS. Sci. World J. 2011, 11, 1865–1876. [Google Scholar] [CrossRef]
- Rysstad, G.; Abrahamsen, R.K. Formation of volatile aroma compounds and carbon dioxide in yogurt starter grown in cows and goats milk. J. Dairy Res. 1987, 54, 257–266. [Google Scholar] [CrossRef]
- Noda, I.; Ozaki, Y. Principle of Two-Dimensional Correlation Spectroscopy. In Two-Dimensional Correlation Spectroscopy: Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons, Ltd.: The Atrium, Southern Gate, Chichester, West Sussex, UK, 2004; ISBN 9780470012390. [Google Scholar]
- Lakowicz, J.R. Quenching of fluorescence. In Principles of Fluorescence Spectroscopy; Springer Science+Business Media, LLC: New York, NY, USA, 1999; pp. 237–265. ISBN 9781475730630. [Google Scholar]
- Herbert, S.; Riaublanc, A.; Bouchet, B.; Gallant, D.J.; Dufour, E. Fluorescence Spectroscopy Investigation of Acid- or Rennet-Induced Coagulation of Milk. J. Dairy Sci. 1999, 82, 2056–2062. [Google Scholar] [CrossRef]
- Dufour, E.; Devaux, M.F.; Fortier, P.; Herbert, S. Delineation of the structure of soft cheeses at the molecular level by fluorescence spectroscopy—Relationship with texture. Int. Dairy J. 2001, 11, 465–473. [Google Scholar] [CrossRef]
- Dufour, E.; Lopez, C.; Riaublanc, A.; Mouhous Riou, N. La spectroscopie de fluorescence frontale: Une approche non invasive de la structure et des interactions entre les constituants des aliments. Agoral 1998, 10, 209–215. [Google Scholar]
- Fox, J.J.B.; Thayer, D.W. Radical oxidation of riboflavin. Int. J. Vitam. Nutr. Res. 1998, 68, 174–180. [Google Scholar] [PubMed]
- Christensen, J.; Becker, E.M.; Frederiksen, C.S. Fluorescence spectroscopy and PARAFAC in the analysis of yogurt. Chemom. Intell. Lab. Syst. 2005, 75, 201–208. [Google Scholar] [CrossRef]
- Kikugawa, K.; Beppu, M. Involvement of lipid oxidation products in the formation of fluorescent and cross-linked proteins. Chem. Phys. Lipids 1987, 44, 277–296. [Google Scholar] [CrossRef]
- Wold, P.J.; Veberg, A.; Lundby, F.; Nikolai, A.; Moan, J. Influence of storage time and color of light on photooxidation in cheese : A study based on sensory analysis and fluorescence spectroscopy. Int. Dairy J. 2006, 16, 1218–1226. [Google Scholar] [CrossRef]
- Abbas, K.; Karoui, R.; Aït-Kaddour, A. Application of synchronous fluorescence spectroscopy for the determination of some chemical parameters in PDO French blue cheeses. Eur. Food Res. Technol. 2012, 234, 457–465. [Google Scholar] [CrossRef]
- Sun, S.Q.; Zhou, Q.; Qin, Z. Atlas of Two-Dimensional Correlation Infrared Spectroscopy for Traditional Chinese Medicine Identification; Chemical Industry Press: Beijing, China, 2003. [Google Scholar]
- Dalgleish, D.G.; Brinkhuis, J.; Payens, T.A.J. The Coagulation of Differently Sized Casein Micelles by Rennet. Eur. J. Biochem. 1981, 119, 257–261. [Google Scholar] [CrossRef]



| Sign of Identified Peak | |||||
|---|---|---|---|---|---|
| Peak Position (nm) | 298 nm | 321 nm | 360 nm | 403 nm | 450 nm |
| 298 nm | + | + | + | - | + |
| (-) | (+) | (-) | (+) | ||
| 322 nm | + | + | - | + | |
| (+) | (-) | (+) | |||
| 360 nm | + | - | + | ||
| (-) | |||||
| 403 nm | + | + | |||
| (+) | |||||
| 450 nm | + | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boukria, O.; El Hadrami, E.M.; Sultanova, S.; Safarov, J.; Leriche, F.; Aït-Kaddour, A. 2D-Cross Correlation Spectroscopy Coupled with Molecular Fluorescence Spectroscopy for Analysis of Molecular Structure Modification of Camel Milk and Cow Milk Mixtures during Coagulation. Foods 2020, 9, 724. https://doi.org/10.3390/foods9060724
Boukria O, El Hadrami EM, Sultanova S, Safarov J, Leriche F, Aït-Kaddour A. 2D-Cross Correlation Spectroscopy Coupled with Molecular Fluorescence Spectroscopy for Analysis of Molecular Structure Modification of Camel Milk and Cow Milk Mixtures during Coagulation. Foods. 2020; 9(6):724. https://doi.org/10.3390/foods9060724
Chicago/Turabian StyleBoukria, Oumayma, El Mestafa El Hadrami, Shaxnoza Sultanova, Jasur Safarov, Françoise Leriche, and Abderrahmane Aït-Kaddour. 2020. "2D-Cross Correlation Spectroscopy Coupled with Molecular Fluorescence Spectroscopy for Analysis of Molecular Structure Modification of Camel Milk and Cow Milk Mixtures during Coagulation" Foods 9, no. 6: 724. https://doi.org/10.3390/foods9060724