Abstract
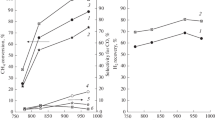
Steam reforming of n-butane has been studied in a membrane reactor at temperatures of 673–823 K, feed hourly space velocities of 1800 and 3600 h–1, and steam/butane ratios of 3, 5 and 7. Under selected conditions, the steam conversion is accompanied by the formation of carbon deposits, whose yield decreases with increasing steam excess. However, with an increase in steam excess, its negative effect on H2 recovery through the membrane increases. Comparative experiments with the “non-membrane” reaction at a constant steam/butane ratio equal to 5 have shown that butane conversion to the target products increases in the membrane reactor and the rate of carbon deposits formation decreases. When replacing the sweep gas by the vacuum conditions in the permeate side, there is a further decrease in the rate of carbon deposits formation and an increase in the butane conversion in the main reaction. With an increase in feed hourly space velocity from 1800 to 3600 h–1, the rate of carbon deposits formation and its dependence on temperature increase. At an hourly space velocity of 1800 h–1, the rate of formation of carbon deposits is two to three times lower and varies slightly with temperature. Under these conditions, the butane conversion is close to 100%; about 80% of H2 formed is recovered from the reaction mixture, and at vacuum conditions in the permeate side, the H2 recovery is 95%.







Similar content being viewed by others
REFERENCES
N. Laosiripojana, W. Sutthisripok, S. Charojrochkul, and S. Assabumrungrat, Fuel 90, 136 (2011).
C. N. Avila-Neto, K. D. Oliveira, K. F. Oliveira, M. M. Aline, A. M. M. Arouca, R. A. R. Ferreira, and C. E. Hori, Appl. Catal., A 550, 184 (2018).
L. Zhang, X. Wang, B. Tan, and U. S. Ozkan, J. Mol. Catal. A: Chem. 297, 26 (2009).
K. M. Kim, B. S. Kwak, N. K. Park, T. J. Lee, S. T. Lee, and M. J. Kang, Ind. Eng. Chem. 46, 324 (2017).
E. C. Faria, R. C. Rabelo-Neto, R. C. Colman, R. A. R. Ferreira, C. E. Hori, and F. B. Noronha, Catal. Lett. 146, 2229 (2016).
N. Laosiripojana, W. Sangtongkitcharoen, and S. Assabumrungrat, Fuel, 323 (2006).
V. M. Gryaznov, Dokl. Akad. Nauk SSSR 189, 794 (1969).
V. M. Gryaznov, Platinum Met. Rev. 30, 68 (1986).
A. P. Mishchenko, V. M. Gryaznov, and M. E. Sarylova, Russ. Chem. Bull., Int. Ed. 40, 1149 (1991).
V. M. Gryaznov, M. M. Ermilova, N. V. Orekhova, and E. V. Skakunova, Russ. Chem. Bull., Int. Ed. 37, 637 (1988).
V. M. Gryaznov, E. V. Skakunova, and M. M. Ermilova, Russ. Chem. Bull., Int. Ed. 37, 858 (1988).
V. M. Gryaznov, Sep. Purif. Rev. 29, 171 (2000).
E. Drioli, A. I. Stankievicz, and F. Macedonian, J. Membr. Sci. 380, 1 (2011).
A. A. Lytkina, N. V. Orekhova, and A. B. Yaroslavtsev, Petr. Chem. 58, 911 (2018).
E. Yu. Mironova, M. M. Ermilova, N. V. Orekhova, D. N. Muraviev, and A. B. Yaroslavtsev, Catal. Today 236, 64 (2014).
V. V. Volkov, E. G. Novitskii, G. A. Dibrov, P. V. Samokhin, M. A. Kipnis, and A. B. Yaroslavtsev, Catal. Today 193, 31 (2012).
L. P. Didenko, V. I. Savchenko, L. A. Sementsova, P. E. Chizhov, L. A. Bykov, Petr. Chem. 53, 27 (2013).
L. P. Didenko, V. I. Savchenko, L. A. Sementsova, and P. E. Chizhov, Petr. Chem. 56, 459 (2016).
N. V. Orekhova, L. M. Kustov, A. V. Kucherov, E. D. Finashina, M. M. Ermilova, and A. B. Yaroslavtsev, Nanotechnol. Russ, 7, 560 (2012).
Y. Guo, G. Lu, Y. Wang, and R. Wang, Sep. Purif. Technol. 32, 271 (2003).
L. M. Neal, S. Yusuf, J. A. Sofranko, and F. Li, Energy Technol. 4, 1200 (2016).
M. L. Rodriguez, D. Ardissone, E. Heracleous, A. Lemo-nidon, E. Lopez, M. N. Pedernera, and D. O. Borio, Catal. Today 157, 303 (2010).
M. Ermilova, A. Kucherov, N. Orekhova, E. Finashina, L. Kustov, and A. Yaroslavtsev, Chem. Eng. Process: Process Intensif. 126, 150 (2018).
M. V. Tsodikov, A. S. Fedotov, D. O. Antonov, V. I. Uvarov, and F. C. Luck, Int. J. Hydrogen Energy 41, 2424 (2016).
A. S. Fedotov, D. O. Antonov, O. V. Bukhtenko, V. I. Uvarov, and M. V. Tsodikov, Int. J. Hydrogen Energy 42, 24 131 (2017).
M. V. Tsodikov, A. S. Fedotov, D. O. Antonov, V. I. Uvarov, and S. N. Khadzhiev, RF Patent 2638350, Byull. Izobret. No. 2 (2017).
A. Basile, S. Campanari, G. Manzolini, A. Iulianelli, T. Longo, S. Liguori, M. De Falco, and V. Piemonte, Int. J. Hydrogen Energy 36 (2), 1531 (2011).
Y. Shirasaki, T. Tsuneki, Y. Ota, I. Yasuda, S. Tachibana, H. Nakajima, K. Kobayashi, Int. J. Hydrogen Energy 34, 4482 (2009).
K. Takao, I. Yoichi, I. Takaya, Y. Hisataka, T. Hiroyuki, H. Hideaki, T. Yasuhiro, and I. Masaya, Int. J. Hydrogen Energy 38, 6079 (2013).
T. Boeltken, A. Wunsch, T. Gietzelt, P. Pfeifer, and R. Dittmeyer, Int. J. Hydrogen Energy 39, 18 058 (2014).
M. Maki, H. Mitoki, and I. Tatsumi, Int. J. Hydrogen Energy 38, 6673 (2013).
Panyuan Li, Zhi Wang, Zhihua Qiao, Yanni Liu, Xiaochang Cao, Wen Li, Jixiao Wang, and Shichang Wang, J. Membr. Sci. 495, 130 (2015).
G. Burkhanov, N. Gorina, N. Kolchugina, and N. Roshan, Platinum Met. Rev. 55, 3 (2011).
L. P. Didenko, V. I. Savchenko, L. A. Sementsova, and L. A. Bykov, Int. J. Hydrogen Energy 41, 307 (2016).
Y. S. Cheng and K. L. Yeung, J. Membr. Sci. 182, 195 (2001).
L. P. Didenko, L. A. Sementsova, P. E. Chizhov, V. N. Babak, and V. I. Savchenko, Russ. Chem. Bull., Int. Ed. 6, 1997 (2016).
L. P. Didenko, L. A. Sementsova, P. E. Chizhov, and T. V. Dorofeeva, Petr. Chem. 59, 394 (2019).
Kh. Kramers and K. Vestertern, Chemical Reactors (Khimiya, Moscow, 1967) [in Russian].
V. N. Babak, L. P. Didenko, and Yu. P. Kvurt, in Proceedings of the International Conference on Mathematical Methods in Engineering and Technology MMTT-32, St. Petersburg,2019 [in Russian].
Y.-M. Lin, S.-L. Liu, C.-H. Chuang, and Y.-T. Chu, Catal. Today 82, 127 (2003).
F. Gallucci, A. Comite, G. Capannelli, and A. Basile, Ind. Eng. Chem. Res. 45, 2994 (2006).
M. Falko and Paola L. Marrelli, Int. J. Hydrogen Energy 32, 2094 (2007).
А. Iulianelli, G. Manzolini, M. De Falco, S. Campanari, T. Longo, S. Liguori, and A. Basele, Int. J. Hydrogen Energy 35, 11 514 (2010).
H. Butcher, C. J. E. Quenzel, L. Breziner, J. Mettes, B. A. Wilhite, and P. Bossar, Int. J. Hydrogen Energy, 111 (2014).
X. Wang and R. J. Gorte, Catal. Lett. 73, 15 (2001).
A. Igarashi, T. Ohtaka, and S. Motoki, Catal. Lett. 13, 189 (1991).
M. M. Zyryanova, P. V. Snytnikov, Yu. I. Amosov, V. D. Belyaev, and V. A. Sobyanin, Fuel 108, 282 (2013).
M. M. Zyryanova, S. D. Badmaev, V. D. Belyaev, Yu. I. Amosov, P. V. Snytnikov, V. A. Kirillov, and V. A. Sobyanin, Catal. Industry 5, 312 (2013).
M. M. Zyryanova, P. V. Snytnikov, A. B. Shigarov, V. D. Belyaev, V. A. Kirillov, and V. A. Sobyanin, Fuel 135, 76 (2014).
ACKNOWLEDGMENTS
This work was carried out as part of the Program of Fundamental Scientific Research of the State Academies of Sciences for 2013–2020, no. 0089-2014-0032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Didenko, L.P., Sementsova, L.A., Babak, V.N. et al. Steam Reforming of n-Butane in Membrane Reactor with Industrial Nickel Catalyst and Foil Made of Pd-Ru Alloy. Membr. Membr. Technol. 2, 85–97 (2020). https://doi.org/10.1134/S2517751620020055
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751620020055