Abstract
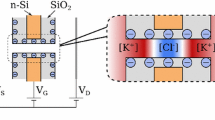
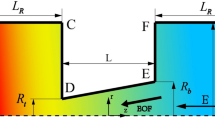
An interesting area of modern membrane science is the development of “smart” membranes, which can affect the transport properties of selected components via external tuning. When the target components are ions, such a tuning can be realized with the help of electric field created by the conductive pore surface. We have proposed in this work a mathematical model of ions transport in a cylindrical nanopore with the electronic charge at the conductive surface and the chemical charge, which is separated from the surface by the Stern layer. The model is based on one-dimensional equations for potential, ion concentrations, and pressure in the diffuse layer. It is applied for describing the membrane potential at zero current, which characterizes the type and strength of ionic selectivity. It is shown that the change of surface potential in the direction from negative to positive results in the continuous change of pore selectivity from cation to anion. The decrease in the Stern layer capacitance and increase in the pore radius lead to the decrease in ionic selectivity. The presence of positive (negative) chemical charge causes the shift of potential value, at which the selectivity is switched, in the direction of negative (positive) values. At this value of potential, the membrane becomes non-selective, the diffusive flux of ions reaches maximum, and the osmotic flow ceases. The suggested model provides a qualitative and quantitative description of experimental results on switchable selectivity of track-etched membranes modified by the gold coating.







Similar content being viewed by others
REFERENCES
L. F. Greenlee, D. F. Lawler, B. D. Freeman, B. Marrot, and P. Moulin, Water Res. 43, 2317 (2009).
V. V. Volkov, B. V. Mchedlishvili, V. I. Roldugin, S. S. Ivanchev, and A. B. Yaroslavtsev, Nanotechnol. Russ. 3, 656 (2008).
H. Strathmann, Desalination 264, 268 (2010).
S. Porada, R. Zhao, A. Van der Wal, V. Presser, and P. M. Biesheuvel, Prog. Mater. Sci. 58, 1388 (2013).
Y. Tanaka, Ion Exchange Membranes: Fundamentals and Applications (Elsevier, Amsterdam, 2015).
A. B. Yaroslavtsev and V. V. Nikonenko, Nanotechnol. Russ. 4, 137 (2009).
P. Yu. Apel, O. V. Bobreshova, A. V. Volkov, V. V. Volkov, V. V. Nikonenko, I. A. Stenina, A. N. Filippov, Yu. P. Yampolskii, and A. B. Yaroslavtsev, Membr. Membr. Technol. 1, 45 (2019).
R. W. Baker, Membrane Technology and Applications (England: John Wiley & Sons, Chichester, 2004).
J. Bobacka, A. Ivaska, and A. Lewenstam, Chem. Rev. 108, 329 (2008).
W. Sparreboom, A. Berg, and J. C. T. Eijkel, Nature Nanotech. 4, 713 (2009).
L. Zhang, S. R. Chae, Z. Hendren, J. S. Park, and M. R. Wiesner, Chem. Eng. J. 204–206, 87 (2012).
M. Tagliazucchi and I. Szleifer, Mater. Today 18, 131 (2015).
Z. S. Siwy and S. Howorka, Chem. Soc. Rev. 39, 1115 (2010).
X. Hou, W. Guo, and L. Jiang, Chem. Soc. Rev. 40, 2385 (2011).
P. Yu. Apel, I. V. Blonskaya, N. V. Levkovich, and O. L. Orelovich, Petr. Chem. 51, 555 (2011).
M. Nishizawa, V. P. Menon, and C. R. Martin, Science 268, 700 (1995).
C. R. Martin, M. Nishizawa, K. Jirage, M. Kang, and S. B. Lee, Adv. Mater. 13, 1351 (2001).
M. Kang and C. R. Martin, Langmuir 17, 2753 (2001).
P. Gao and C. R. Martin, ACS Nano 8, 8266 (2014).
D. V. Lebedev, A. V. Shiverskiy, M. M. Simunin, V. S. Solodovnichenko, V. A. Parfenov, V. V. Bykanova, S. V. Khartov, and I. I. Ryzhkov, Petr. Chem. 57, 306 (2017).
V. S. Solodovnichenko, D. V. Lebedev, V. V. Bykanova, A. V. Shiverskiy, M. M. Simunin, V. A. Parfenov, and I. I. Ryzhkov, Adv. Eng. Mater. 19, 1700244 (2017).
D. V. Lebedev, V. S. Solodovnichenko, M. M. Simunin, and I. I. Ryzhkov, Petr. Chem. 58, 474 (2018).
W. Guan and M. A. Reed, Nano Lett. 12, 6441 (2012).
H. Zhang, X. Quan, X. Fan, C. Yi, S. Chen, H. Yu, and Y. Chen, Environ. Sci. Technol. 53, 868 (2019).
D. Mattia, H. Leese, and K. P. Lee, J. Membr. Sci. 475, 266 (2015).
M. Alsawat, T. Altalhi, T. Kumeria, A. Santos, and D. Losic, Carbon 93, 681 (2015).
M. Sarno, A. Tamburrano, L. Arurault, S. Fontorbes, R. Pantani, L. Datas, P. Ciambelli, and M. S. Sarto, Carbon 55, 10 (2013).
P. Ramírez, S. Mafé, A. Alcaraz, and J. Cervera, J. Phys. Chem. B 107, 13178 (2003).
C. Amatore, A. I. Oleinick, and I. Svir, Chem. Phys. Chem. 10, 211 (2009).
A. N. Filippov, Colloid J. 80, 716 (2018).
R. J. Gross and J. F. Osterle, J. Chem. Phys. 49, 228 (1968).
P. B. Peters, R. Van Roij, M. Z. Bazant, and P. M. Biesheuvel, Phys. Rev. E. 93, 053108 (2016).
I. I. Ryzhkov, D. V. Lebedev, V. S. Solodovnichenko, A. V. Shiverskiy, and M. M. Simunin, Phys. Rev. Lett. 119, 226001 (2017).
I. I. Ryzhkov, D. V. Lebedev, V. S. Solodovnichenko, A. V. Minakov, and M. M. Simunin, J. Membr. Sci. 549, 616 (2018).
I. I. Ryzhkov, A. S. Vyatkin, and A. V. Minakov, J. Sib. Fed. Univ.: Math. Phys. 11, 494 (2018).
I. I. Ryzhkov, A. S. Vyatkin, and M. I. Medvedeva, J. Sib. Fed. Univ.: Math. Phys. 12, 579 (2019).
L. Zhang, P. M. Biesheuvel, and I. I. Ryzhkov, Phys. Rev. Appl. 12, 014039 (2019).
J. Duval, J. Lyklema, J. M. Kleijn, and H. P. Van Leeuwen, Langmuir 17, 7573 (2001).
J. Duval, J. M. Kleijn, J. Lyklema, and H. P. Van Leeuwen, J. Electroanal. Chem. 532, 337 (2002).
C. H. Hamman, A. Hamnett, and W. Veilstich, Electrochemistry (Wiley-WCH, 2007).
V. M. Dorokhov, G. A. Martynov, V. M. Starov, and N. V. Churaev, Kolloidn. Zh. 46, 651 (1984).
V. M. Dorokhov, G. A. Martynov, V. M. Starov, and N. V. Churaev, Kolloidn. Zh. 46, 1088 (1984).
M. D. Porter, T. B. Bright, D. L. Allara, and C. E. D. Chidsey, J. Am. Chem. Soc. 109, 3559 (1987).
S. Rentsch, H. Siegenthaler, and G. Papastavrou, Langmuir 23, 9083 (2007).
Funding
This work was financially supported by the Russian Foundation for Basic Research, project no. 18-38-20046 mol_a_ved.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated by V. Avdeeva
Rights and permissions
About this article
Cite this article
Ryzhkov, I.I., Vyatkin, A.S. & Mikhlina, E.V. Modelling of Conductive Nanoporous Membranes with Switchable Ionic Selectivity. Membr. Membr. Technol. 2, 10–19 (2020). https://doi.org/10.1134/S2517751620010072
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S2517751620010072